Introduction
Remdesivir is a nucleotide prodrug whose active metabolite inhibits viral RNA-dependent RNA polymerases that play a vital role in the replication of a broad range of viruses, including Coronaviridae. It has demonstrated clinical benefit in a placebo-controlled trial in patients with severe coronavirus disease 2019 (COVID-19); however, its effect in patients with the moderate disease is unknown.
Aim
To evaluate the efficacy and adverse events of remdesivir administered for 5- or 10-days vs standard care in hospitalized patients with moderate COVID-19
Patient profile
- Hospitalized patients with SARS-CoV-2 infection confirmed by polymerase chain reaction assay within 4 days of randomization and moderate COVID-19 pneumonia
- Defined as any radiographic evidence of pulmonary infiltrates and oxygen saturation >94% on room air
Methods
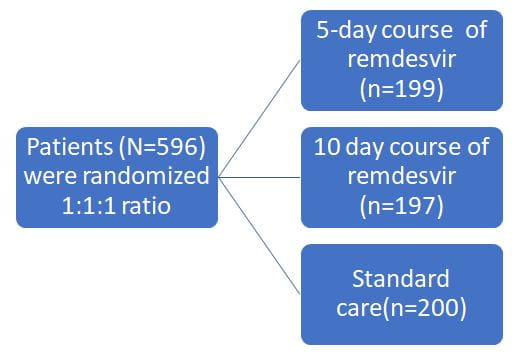
- Randomized, open-label, phase 3 trial
- Enrolled patients from 105 hospitals in the United States, Europe, and Asia between March 15, 2020, and April 18, 2020
200 mg of remdesivir intravenously on day 1, followed by 100 mg of remdesivir once daily for the subsequent days, infused over 30 to 60 minutes
Study Endpoints
- The primary efficacy endpoint was the distribution of clinical status assessed on the 7-point ordinal scale on study day 11
- The secondary endpoint was the proportion of patients with adverse events throughout the study
Results
|
Characteristics |
10-Day remdesivir (n = 193) |
5-Day remdesivir (n = 191) |
Standard care (n = 200) | |
|
Age, median (IQR), y |
56 (45-66) |
58 (48-66) |
57 (45-66) | |
|
Sex, No. (%) |
|
|
| |
|
Male |
118 (61) |
114 (60) |
125 (63) | |
|
Female |
75 (39) |
77 (40) |
75 (38) | |
|
Race, No./total (%) |
|
|
| |
|
White |
107/188 (57) |
109/186 (59) |
112/193 (58) | |
|
Black |
37/188 (20) |
35/186 (19) |
27/193 (14) | |
|
Asian |
31/188 (16) |
34/186 (18) |
37/193 (19) | |
|
Othera |
13/188 (7) |
8/186 (4) |
17/193 (9) | |
|
Hispanic or Latino ethnicity, |
42/186 (23) |
25/187 (13) |
34/186 (18) | |
|
No./total (%)b |
|
|
| |
|
Body mass index, median (IQR)c |
28 (25-32) |
27 (24-30) |
27 (24-31) | |
|
Day 1 clinical status on 7-point scale, No. (%) |
|
|
| |
|
3: Hospitalized, requiring noninvasive ventilation or high-flow oxygen |
1 (1) |
2 (1) |
2 (1) | |
|
4: Hospitalized, requiring low-flow supplemental oxygen |
23 (12) |
29 (15) |
36 (18) | |
|
5: Hospitalized, not requiring supplemental oxygen but requiring ongoing medical care |
163 (84) |
160 (84) |
160 (80) | |
|
6: Hospitalized, not requiring supplemental oxygen or ongoing medical cared |
6 (3) |
0 |
2 (1) | |
|
Coexisting conditions, No. (%) |
|
|
| |
|
Cardiovascular disease |
111 (58) |
111 (58) |
107 (54) | |
|
Hypertension |
85 (44) |
82 (43) |
81 (41) | |
|
Diabetes |
85 (44) |
71 (37) |
76 (38) | |
|
Asthma |
31 (16) |
22 (12) |
28 (14) | |
|
Duration of hospitalization before first dose of remdesivir, median (IQR), d |
2 (1-3) |
2 (1-3) |
2 (1-3) | |
|
Duration of symptoms before first dose of remdesivir, median (IQR), d |
8 (5-11) |
8 (5-11) |
9 (6-11) | |
|
Concomitant medications, No. (%)e |
|
|
| |
|
Steroids |
29 (15) |
33 (17) |
38 (19) | |
|
Hydroxychloroquine/chloroquine |
22 (11) |
16 (8) |
89 (45) | |
|
Lopinavir-ritonavir |
11 (6) |
10 (5) |
43 (22) | |
|
Tocilizumab |
1 (1) |
1 (1) |
10 |
(5) |
|
Azithromycin |
41 (21) |
35 (18) |
62 (31) | |
|
Aspartate aminotransferase, median (IQR), U/L |
34 (23-48) |
32(25-48) |
34 (24-49) | |
|
Alanine aminotransferase, median (IQR), U/L |
28 (21-47) |
30 (19-51) |
30 (19-49) | |
|
Estimated glomerular filtration rate, median (IQR), mL/minf |
110 (86-143) |
99 (75-130) |
103 (78-130) | |
IQR, interquartile range.
a Includes American Indian or Alaska Native, Native Hawaiian or Pacific Islander, Arab, unknown, and not specified.
b In patients with available ethnicity data.
c Calculated as weight in kilograms divided by height in meters squared.
d Some patients remained hospitalized for quarantine purposes or other social issues even if they did not require medical care.
e Includes medications taken between first and last dose of remdesivir (or after day 1 for the standard care group).
f Glomerular filtration rate estimated by the Cockcroft-Gault formula.
- Overall, 56% of patients had cardiovascular disease, 42% had hypertension, 40% had diabetes, and 14% had asthma
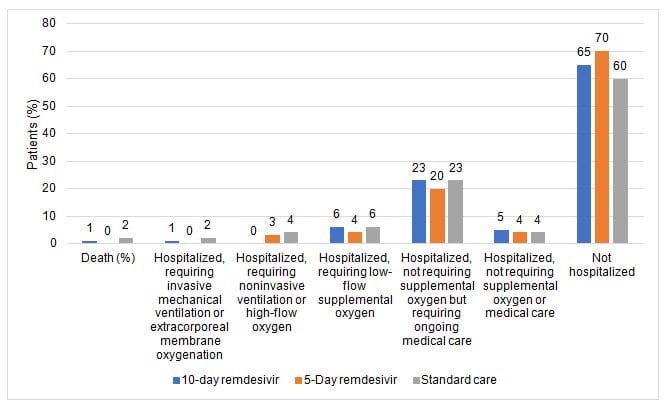
- All patients had an oxygen saturation above 94% while breathing room air at screening
- On day 11, patients in the 5-day remdesivir group had statistically significantly higher odds of a better clinical status distribution than those receiving standard care (odds ratio, 1.65; 1.09-2.48; P = .02).
|
|
10-day remdesivir |
5-day remdesivir |
Standard care |
|
Primary endpoint: difference in clinical status distribution vs standard care, odds ratioa |
|
1.65 |
1 [Reference] |
|
P value |
.18 |
.02 |
|
|
Clinical improvement, No. (%)b |
|
|
|
|
Day 5 |
72 (37) |
61 (32) |
66 (33) |
|
Day 7 |
92 (48) |
106 (56) |
94 (47) |
|
Day 11 |
126 (65) |
134 (70) |
121 (61) |
|
Difference in percentage vs standard care at day 11 |
4.8 |
9.7 |
|
|
Day 14 |
148 (77) |
146 (76) |
135 (68) |
|
Day 28 |
174 (90) |
171 (90) |
166 (83) |
|
Recovery, No. (%) c |
|
|
|
|
Day 5 |
74 (38) |
67 (35) |
71 (36) |
|
Day 7 |
94 (49) |
114 (60) |
101 (51) |
|
Day 11 |
132 (68) |
141 (74) |
128 (64) |
|
Difference in percentage vs standard care at day 11 |
4.4 |
9.8 |
|
|
Day 14 |
153 (79) |
153 (80) |
145 (73) |
|
Day 28 |
178 (92) |
175 (92) |
170 (85) |
a The odds ratio and P-value for the 5-day remdesivir treatment group comparison were estimated using the proportional odds model. The proportional odds assumption was not met for the 10-day remdesivir group comparison, so no odds ratio is presented; the P-value was calculated using the Wilcoxon rank sum test.
b An improvement of at least 2 points from baseline on the 7-point ordinal scale.
c An improvement from a baseline score of 2 to 5 to a score of 6 or 7 or from a baseline score of 6 to a score of 7
Adverse events (Secondary endpoints)
- Adverse events were experienced by
- 5-day remdesivir group: 51% of patients
- 10-day remdesivir group: 59% of patients
- Standard care group: 47% of patients
- Common adverse events observed in remdesivir group vs standard care group
- Nausea (10% vs 3%)
- Hypokalemia (6%vs 2%)
- Headache (5%vs 3%)
Conclusion
- The study demonstrated that patients with moderate COVID-19 treated with a 10-day course of remdesivir did not have a statistically significant difference in clinical status compared with standard care at 11 days after initiation of treatment.
- Patients randomized to a 5-day course of remdesivir had a statistically significant difference in clinical status compared with standard care, but the difference was of uncertain clinical importance.
Reference
JAMA. August 21, 2020. doi:10.1001/jama.2020.16349

.svg?iar=0&updated=20230109065058&hash=B8F025B8AA9A24E727DBB30EAED272C8)