Introduction
Modified XELIRI (mXELIRI; capecitabine plus irinotecan) regimen has shown promising efficacy and tolerability profiles in the first-line and second-line settings.
Aim
To compare the efficacy and safety of the mXELIRI regimen with that of standard FOLFIRI (leucovorin, fluorouracil, and irinotecan), with or without bevacizumab in both regimens, as second-line therapy for metastatic colorectal cancer.
Patient Profile
Patients who were aged 20 years or older with histologically confirmed and unresectable colorectal adenocarcinoma, and who had withdrawn from first-line chemotherapy for metastatic colorectal cancer
Methods
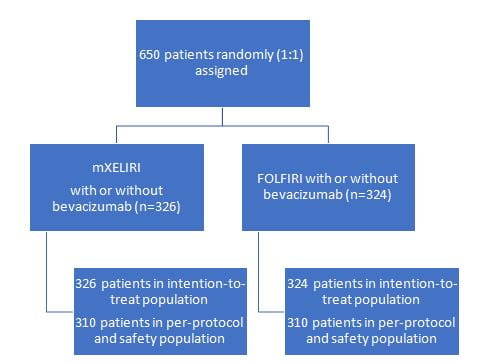
- Multicentre, open-label, randomised, noninferiority, phase 3 trial in 98 hospitals from three countries: Japan (n=73), South Korea (n=8), and China (n=17)
Study Design
16 patients in the mXELIRI group and 14 in the FOLFIRI group were identified as ineligible after enrolment or did not receive any study treatment
Study Regimen
- mXELIRI: Irinotecan 200 mg/m² intravenously on day 1 plus oral capecitabine 800 mg/m² twice daily on days 1−14, repeated every 21 days, with or without bevacizumab 7·5 mg/kg intravenously on day 1
- FOLFIRI with or without: Irinotecan 180 mg/m² intravenously on day 1, leucovorin 200 mg/m² intravenously on day 1, fluorouracil 400 mg/m² intravenously on day 1, and a 46-h continuous intravenous infusion of fluorouracil [2400 mg/m²], repeated every 14 days, with or without the addition of bevacizumab 5 mg/kg intravenously on day 1
Study Endpoint
- The primary endpoint was overall survival, defined as the time from the date of randomisation to the date of death from any cause.
- Secondary endpoints were
- progression-free survival (from the date of randomisation to the date of confirmed progression or death from any cause, whichever occurred first)
- time to treatment failure (from the date of randomisation to the date of confirmed progression, death from any cause, or discontinuation of protocol treatment, whichever occurred first)
- overall response (the proportion of eligible patients with measurable lesions at baseline with a best overall response of complete response or partial response)
- disease control (proportion of eligible patients with measurable lesions with a best overall response of complete response, partial response, or stable disease)
- relative dose intensity (the ratio of delivered dose intensity of chemotherapy to dose intensity planned per protocol)
- incidence of adverse events, and the association between UGT1A1 polymorphisms (UGT1A1*28 or UGT1A1*6, or both) and the incidence of adverse events
Results
- Baseline characteristics were well-balanced between the treatment groups
- The median follow-up was 15.8 months
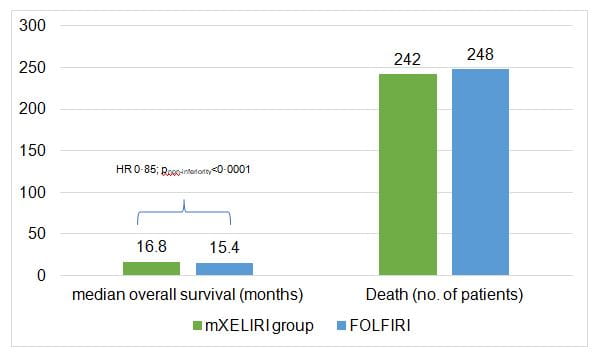
- mXELIRI with or without bevacizumab was non-inferior to FOLFIRI with or without bevacizumab as second-line therapy in metastatic colorectal cancer in terms of overall survival at the predefined noninferiority upper margin of 1·30 (figure 1)
- No significant differences were detected in progression-free survival, time to treatment failure, overall response, or disease control between the treatment groups
- The mXELIRI group showed a manageable tolerability profile that was comparable with that of the FOLFIRI group
- Neutropenia was the most common grade 3–4 adverse event affecting 52 [17%] of 310 patients in the mXELIRI group and 133 [43%] of 310 in the FOLFIRI group
- In mXELIRI group, incidences of grade 3–4 diarrhoea were higher (22 [7%]) than in the FOLFIRI group (ten [3%])
- 310 [46(15%)] patients in the mXELIRI group and 63 (20%) of 310 in the FOLFIRI group reported serious adverse events
- Two treatment-related deaths (one pneumonitis and one lung infection) were observed in the mXELIRI group, and there was one treatment-related death (lung infection) in the FOLFIRI group
Conclusion
- The study demonstrated that overall survival with mXELIRI with or without bevacizumab was non-inferior to that with FOLFIRI with or without bevacizumab in the second-line treatment of patients with metastatic colorectal cancer.
- The findings suggested that mXELIRI might be an effective, well-tolerated, and more convenient alternative to FOLFIRI as a standard second-line backbone therapy for patients with metastatic colorectal cancer, at least for Asian populations.
References
Lancet Oncol 2018; 19: 660–71

.svg?iar=0&updated=20230109065058&hash=B8F025B8AA9A24E727DBB30EAED272C8)