Introduction
Iron deficiency anaemia (IDA) is a significant cause of maternal mortality in India. Maternal anemia also results in higher perinatal morbidity and mortality and higher infant mortality rate. Oral iron replacement therapy is usually recommended for the management of IDA. However, dissociation of ferrous salts into ferrous ions while passing from stomach to intestine and further oxidation of ferrous into ferric ions leads to gastrointestinal (GI) side effects. Among the available iron formulations, ferrous ascorbate is associated with the highest bioavailability and lowest GI side effects. In India, the available formulations contain 13:87 or 20:80 of iron and ascorbic acid respectively. However, as per EAFUS (“Everything’’ Added to Food in The United States) and USFDA, 16:84 ratio is the most stable form yielding less free ferrous ion, thereby reducing the GI side effects.
Aim
To evaluate the GI tolerability of ferrous ascorbate containing 16:84 ratio of iron and ascorbic acid respectively was compared to a brand containing 13:87 or 20:80 iron and ascorbic acid respectively in healthy subjects.
Method
Study Design
- Phase IV, double-blind, randomized, parallel group trial
Patient Profile
- Healthy adults 18–55 years of age with a body mass index (BMI) of 18.5-24.9 kg/m
- Non-smokers, non-alcoholic consumers and non-tobacco chewers
Treatment Strategy
- A total of 60 healthy adults were recruited who underwent clinical, ECG, X-RAY and laboratory examination including serological tests at visit 1
- At visit 2, they underwent clinical examination along with vital signs
- The cohort was randomized to receive either
- Reference product (R) –ferrous ascorbate (13:87 or 20:80 ratio of iron and ascorbic acid respectively) containing 100 mg elemental iron and 1.1 mg folic acid
- Test product (T) – ferrous ascorbate (16:84 ratio of iron and ascorbic acid respectively) containing 100 mg elemental iron and 1.5 mg folic acid
- Reference 1 product (R1) - ferrous ascorbate (13:87 or 20:80 ratio of iron and ascorbic acid respectively) containing 100 mg elemental iron and 1.5 mg folic acid
- The cohort received the iron ascorbate doses daily for 7 consecutive days (visits 3 to 8)
- The clinical assessment, vital signs and safety outcomes were analyzed at visit 9
- The T formulation was compared to R formulation for further analysis
End Points
Primary Endpoint
- Percentage of subjects suffering from any of the GI side effects such as nausea, vomiting, epigastric burning sensation, black-coloured stools and dyspepsia
Secondary End Points
- Incidence of individual adverse events (AEs)
Results
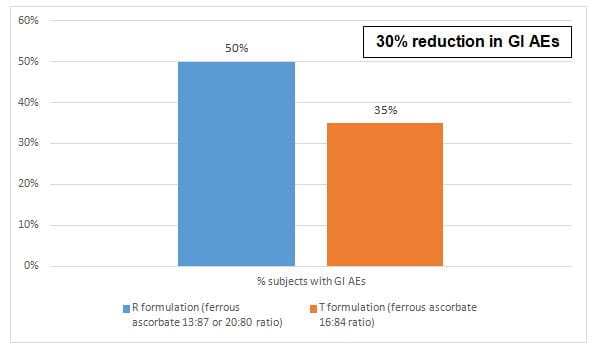
- The incidence of GI side effects was higher in the group who received R formulation as compared to T formulation (p=0.33) as seen in figure 1.
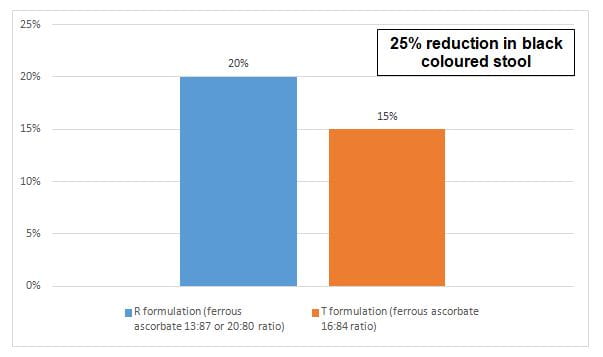
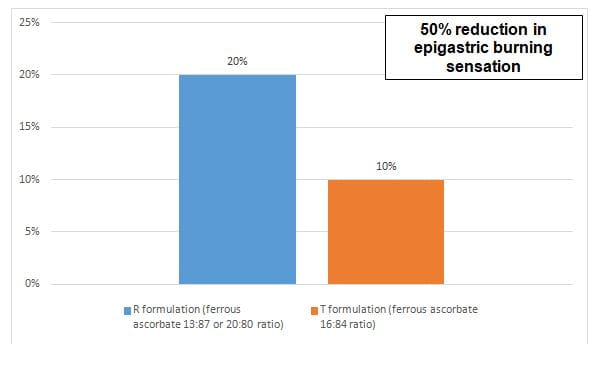
- The percentage of patients reporting individual AEs in both the groups are compared in figure 2 and 3.
Conclusion
- The GI tolerability of ferrous ascorbate containing 16:84 iron: ascorbic acid ratio was better than the conventional formulations containing 13:87 or 20:80 ratio of iron and ascorbic acid respectively, resulting in 30% reduction in the GI side effects and 50% reduction in epigastric burning sensation.
- The absorption of the test product was found to be better than the conventional formulations.
J Pediatr Obstet Gynecol 2010, Sep;1(9):311-16.