Background
The PARADIGM-HF trial has established the safety and tolerability of the target dose of sacubitril/valsartan (200 mg twice daily [97 mg sacubitril and 103 mg valsartan]). Yet, many patients of heart failure with reduced ejection fraction (HFrEF) might not be receiving target doses of ACEI/ARBs in routine practice. This study assessed the impact of the duration of initiation/uptitration regimen on tolerability of sacubitril/valsartan
Aim
To determine the tolerability of initiating/up-titrating sacubitril/valsartan from 50 to 200 mg twice daily (target dose) over 3 and 6 weeks in HFrEF patients (EF ≤35%)
Patient Profile
- Ambulatory or hospitalized HFrEF patients (New York Heart Association [NYHA] class II-IV, EF ≤35%) either treated with a low dose of ACEI/ARB (on a stable dose for last 2 weeks) or ACEI/ARB-na?ve (must not have taken ACEI/ARB for at least 4 weeks) (age ≥18 years;
- Patients need not necessarily have elevated levels of brain natriuretic peptide (BNP) or N-terminal pro-brain natriuretic peptide (NT-proBNP) before enrolling in the study
Methods
Study Design
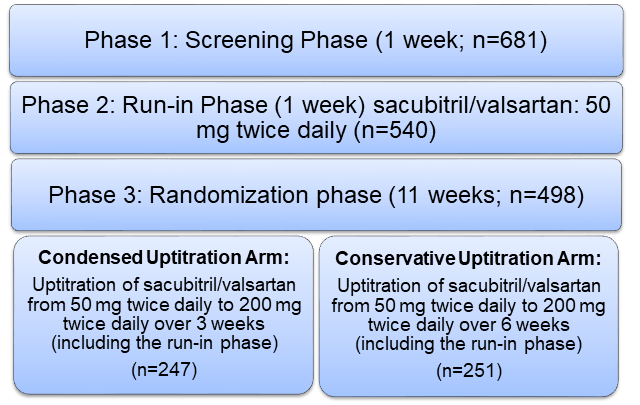
- Multi-centre, randomized, double-blind, parallel-group study conducted in 3 phases
Treatment Strategy
Outcomes
Primary Tolerability Assessments
- The incidence of pre-specified adverse events ([AEs] hypotension, hyperkalemia, renal dysfunction, and angioedema) in both the study arms
- Incidence of systolic blood pressure (SBP) <95 mmHg or pre-specified biochemical changes (serum potassium >5.5 mmol/L and ≥6.0 mmol/L, serum creatinine >3.0mg/dL (267 ?mol/L), and doubling of serum creatinine from baseline levels)
Secondary Outcomes
- Treatment success defined as the proportion of patients (excluding non-AE- or non-death-related discontinuations) who achieved and maintained the dose of sacubitril/valsartan 200 mg twice daily without any dose interruption or down-titration over 12 weeks
- Tolerability success defined as the proportion of patients (excluding patients who discontinued for reasons other than AE or death), who tolerated a dose of sacubitril/valsartan of 200 mg twice daily for at least the final 2 weeks leading to study completion, irrespective of previous dose interruption or down-titration.
Results
- The study was completed by 429 patients (86.1%)
- There was statistically no significant difference in the incidence of pre-defined hypotension, renal dysfunction and hyperkalemia; and adjudicated angioedema between the condensed and the conservative uptitration regimen (Table 1)
|
Parameter |
Condensed Arm |
Conservative Arm |
P value |
|
Hypotension |
9.7% |
8.4% |
0.570 |
|
Renal dysfunction |
7.3% |
7.6% |
0.990 |
|
Hyperkalemia |
7.7% |
4.4% |
0.114 |
|
Adjudicated angioedema |
0.0% |
0.8% |
- |
- Pre-defined systolic blood pressure <95 mmHg was achieved in 8.9% patients randomized to condensed uptitration vs. 5.2% of those in the conservative uptitration arm (P =0.102). The incidence of SBP <95mmHg in each of the ACEI/ARB strata differed as per the uptitration regimen (interaction P =0.0392). Among patients on low doses of ACEI/ARB, greater proportion of patients in the condensed rather than the conservative uptitration regimen achieved SBP <95 mmHg, (14.3% vs. 4.9%, P =0.016).
- Although the number of ACEI/ARB na?ve patients was small in the cohort.
- Pre-defined serum potassium >5.5 mmol/L was 7.3% vs. 4.0% (P =0.097), and serum creatinine >3.0 mg/dL was 0.4% vs. 0% in the condensed vs. the conservative uptitration regimen, respectively.
- As per uptitration regimen, there was a trend of greater treatment success (77.8%) in the condensed arm vs. than in the conservative arm (84.3%) although this did not reach statistical significance (p=0.078).
- Overall the proportion of patients achieving tolerability success was comparable in the condensed as well as the conservative uptitration regiment (83.0% vs. 87.3%, P =0.207).
- Tolerability success was achieved by similar proportion of patients in high-dose ACEI/ARB stratum irrespective of the uptitration regimen (82.6% vs. 83.8%; P =0.783). Greater proportion of patients in the conservative rather than condensed uptitration regimen achieved tolerability success amongst patients in low-dose ACEI/ARB strata (84.9% vs. 73.6%; P =0.030).
Conclusion
- Initiation/up-titration of sacubitril/valsartan from 50 to 200 mg twice daily over a period of 3 or 6 weeks had a tolerability profile similar to that of other HF treatments.
- A more gradual ‘conservative’ uptitration regimen of sacubitril/valsartan yielded a high treatment success rate even in patients taking a low dose (or those na?ve to) ACEI/ARB.
Eur J Heart Failure. 2016; 18: 1193-1202.

.svg?iar=0&updated=20230109065058&hash=B8F025B8AA9A24E727DBB30EAED272C8)