Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive and fatal disease resulting in gradual decline of lung function. It is associated with a poor prognosis having a similar 5-year survival rate as that of several cancers. The efficacy of Pirfenidone has been evaluated in three phase 3 randomized trials. The first trial conducted in Japan concluded that Pirfenidone reduced the decline in forced vital capacity (FVC) and improved the outcome in patients with idiopathic pulmonary fibrosis (IPF). However in the 2 CAPACITY (Clinical Studies Assessing Pirfenidone in Idiopathic Pulmonary Fibrosis) studies that were carried out, the primary endpoint of reduction in FVC was met in only one of them. This necessitated another trial to support pirfenidone in the management of IPF.
Aim
The objective of the ASCEND (Assessment of Pirfenidone to Confirm the Efficacy and Safety in Idiopathic Pulmonary Fibrosis) study was to confirm the effect of pirfenidone in disease progression in IPF patients.
Patient Profile
Patients having a mean age of 40-80 years confirmed with diagnosis of IPF.
Inclusion Criteria
- 50-90% of the predicted FVC (forced vital capacity)
- 30-90% of the predicted carbon monoxide diffusing capacity (DLCO)
- Ratio of forced expiratory volume in 1 second (FEV1) to FVC of 0.80 or more
- 6 min walk test distance of at least 150 m
Method
Study Design
Multinational, randomized, double-blind, placebo-controlled phase-3 study.
Treatment Strategy
Treatment group n = 278 received oral pirfenidone 2403 mg/day for 52 weeks
Placebo group n = 277
Primary End-point
- Change in percentage of predicted FVC from baseline to week 52
Secondary End-points
- Change in 6-min walk distance (6MWD) from baseline to week 52
- Progression-free survival was defined as time to the first occurrence of any of the following –
- Confirmed decrease of 10 percentage points or more in the % predicted FVC
- Confirmed decrease of 50 m or more in 6MWD
- Death
- Change in Dyspnea
- Rate of death from any cause
- Deaths due to IPF during the period from baseline to 28 days after the last dose of the drug
Results
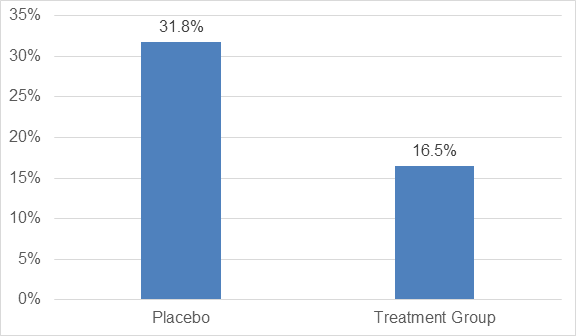
- There was a significant reduction of 47.9% in the proportion of patients with a decline of 10% or more in % predicted FVC or who had died as compared to the placebo group as seen in figure 1.
- The proportion of patients with no decline in % predicted FVC was increased by 132.5% in the treatment group as compared to placebo (p<0.001) as shown in figure 2.
- The change from baseline to week 52 in the 6MWD was significant in the treatment group (p=0.04)
- A decrease of 50 m or more in 6MWD was seen in 25.9% in the treatment group as compared to 35.7% in placebo, with a relative reduction of 27.5% in the pirfenidone group
- Pirfenidone significantly reduced the relative risk of death or disease progression by 43% (p<0.001)
- There was no significant between-group difference in dyspnea scores (p=0.16), rates of death from any cause (p=0.01) and death due to IPF (p=0.23)
- Pre-specified analysis of results from the previous 2 studies showed that there was a significant difference between the 2 groups, favoring pirfenidone, in death from any cause (p=0.01) and deaths due to IPF (p=0.006)
Adverse Events
- Gastrointestinal adverse events was 5.4% in the treatment group vs 1.4% in the placebo group
- 1.8% in treatment group reported skin related adverse events as compared 0.4% in placebo group
- The adverse events rarely led to discontinuation of the treatment
Conclusions
- Treatment with pirfenidone in IPF patients was associated with reduced disease progression
- Treatment with pirfenidone was safe and associated with acceptable side effect profile and fewer deaths
N Engl J Med. 2014 May 29;370(22):2083-92. Doi: 10.1056/NEJMoa1402582.