Authors: V. Naik1, S. Chhowala1, M. Lopez1, R. Hegde1, J. Gogtay1, Geena M2, V. Mhapsekar2, P. Rege2, N. Ransing2
Affiliations: 1. Medical Affairs Cipla Ltd, Mumbai Central, Mumbai, India; 2. Integrated Product Development, Cipla Ltd, Vikhroli, Mumbai, India Introduction
- The Revolizer® is a single dose, pin-based, dry powder inhaler (DPI), which helps to overcome the co-ordination problem seen with the pressurized metered dose inhalers.
- The medication (eg. formoterol/budesonide) is provided as a micronized inhalation powder, formulated with lactose as an excipient and supplied in individual single-dose capsules–Rotacaps®. The intrinsic resistance of the Revolizer® and the inspiratory flow rate generated by the patient on inhalation through the device produces a turbulence which ensures effective de-agglomeration of the inhalation powder.
- Performance of a device is critical in determining optimal drug deposition in the lungs, which in turn impacts treatment outcomes.
- We evaluated the in-vitro performance of the Revolizer® at variable flow rates, using formoterol/budesonide (6/200 mcg) (Foracort rotacaps® 200, Cipla Ltd.)
- In-vitro parameters evaluated included:
- % Fine Particle Mass (FPM) – Quantity of drug in the size range of 1- 5 micron, which has the potential to deposit in the airways, expressed as % of the delivered dose.
- Mass median aerodynamic diameter (MMAD) – Expresses the particle size distribution of an aerosol. It is the diameter at which 50% of the particles of an aerosol by mass are larger and 50% are smaller. For efficient lung deposition, the MMAD should be lower than 5 ?m,
- Delivered dose uniformity (DDU) - The Delivered dose uniformity is the consistency of the total amount of drug emitted from the inhaler device which is available to the patient.
- Resistance - A significant factor to balance achievable flow rates with the efficient utilization of inspiratory air flow for powder dispersion and deagglomeration required for therapeutic effect.
These parameters are Critical Quality Attributes (CQA’s), recommended by international regulatory agencies for determining the safety, quality and efficacy of an orally inhaled product.
Methods
- %FPM and MMAD of the individual active ingredients (formoterol and budesonide) were measured using Next Generation Impactor (NGI, Copley Scientific) at variable flow rates of 30,45, 60, 90 L/min (Figure 1). The NGI measures the amount of drug deposited in the lungs. It is designed to simulate the human lung and comprises of a number of stages/plates with filters arranged in descending order of filter size to mimic the bronchiole size in the lungs.
The Revolizer® device was connected to the NGI and the flow rate was set at 30 L/min, using a vacuum pump attached to the outlet of the NGI. The active substance (formoterol and budesonide) was extracted from the induction port, pre separator and collection plate of each of the stages into an aliquot of solvent and analyzed using high-performance liquid chromatograph (HPLC), followed by calculation of the % FPF and MMAD. The same procedure was repeated for the other flow rates – 45, 60, 90 L/min.
- DDU of the individual active ingredients (formoterol and budesonide) was measured using Dosage Unit Sampling Apparatus (DUSA, Copley Scientific) at a flow rate of 60L/min. (Figure 2).
- The Revolizer® device was connected to the DUSA and the flow rate was set at 60 L/Min using a vacuum pump attached to the collector part of the DUSA. The device was dismantled and the active substance (formoterol and budesonide) was extracted from the apparatus into an aliquot of solvent and analyzed using HPLC, followed by calculation of the delivered dose uniformity.
- Device resistance for Revolizer® was measured using Critical Flow Controller (TPK2000, Copley Scientific). This instrument is interposed between the DUSA and vacuum pump and helps to control flow conditions during testing.
Results
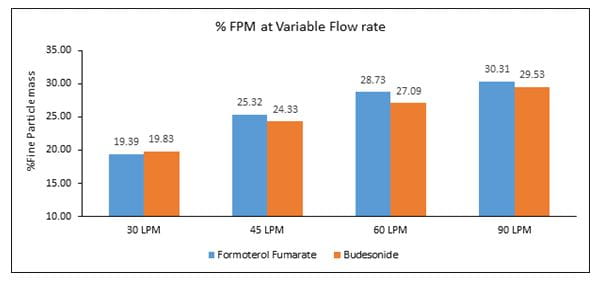
- The %FPM at 30, 45, 60 and 90L/min was 19.39, 25.32, 28.73 and 30.31, respectively for formoterol and 19.83, 24.33, 27.09 and 29.53, respectively for budesonide (Figure 3).
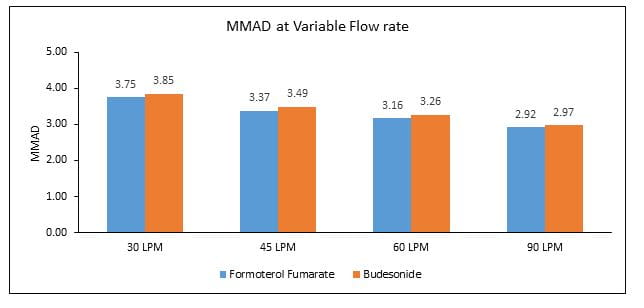
- The MMAD at 30, 45, 60 and 90L/min was 3.75, 3.37, 3.16 and 2.92, respectively for formoterol and 3.85, 3.49, 3.26 and 2.97, respectively for budesonide (Figure 4).
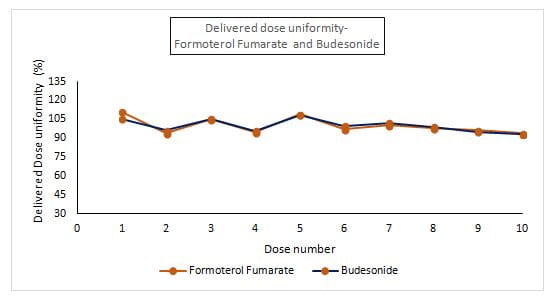
- The DDU was found to be in the range of 93.9%-111.1% for formoterol and 93%-108.1% for budesonide (Figure 5).
- The resistance for Revolizer® was found to be 0.045 kPa0.5L/min at an inspiratory flow rate of approximately 44 L/min.
Discussion
- Ensuring efficient and consistent drug delivery through an inhaler device is a critical element in the development and manufacturing of DPIs, as suboptimal drug delivery could potentially adversely affect disease management
- The Revolizer® showed a higher % FPM for formoterol and budesonide which was in the range of 20 -30 %, indicating optimum drug deposition under in vitro conditions
- The MMAD for formoterol and budesonide when delivered using the Revolizer® was below 5 ?m, which can ensure effective lung deposition
- Also, the % FPM and MMAD for each component did not vary largely across flow rates, indicating constant amount of the respirable fraction (amount of drug which measures less than 5 microns in particle size and has the potential to deposit in the lungs)
- The Revolizer® showed a consistent DDU for the active ingredients which was well within the pharmacopoeial specified limits (Limit: 75%-125%), thus indicating homogeneity in drug quantity across all the Rotacaps in the sample batch tested.
- Higher the resistance, lower is the inhalation effort required to generate the required turbulence within the chamber. The higher resistance of Revolizer® can help it to work even in patients with lower flow rates.
Conclusion
Overall, Revolizer® demonstrated efficient drug delivery across flow rates (30, 45, 60 and 90 L/min) which was shown to be uniform and within the specified limits.
The Revolizer® evaluated using formoterol/budesonide Rotacaps®, delivered effectively and consistently across various flow rates, thus complying with the performance requirements of Indian pharmacopoeia for pharmaceutical-DPI products.
References
- European Respiratory Journal 2014; 44: 4635
- Copley – Scientific – 2019
- JACI 2019; 139 (6): 2013–2014.e1
Poster presented at NAPCON, November 22-24, 2019, Kochi, India