The increasing prevalence of multidrug-resistant Gram-negative bacteria (MDRGNB) especially Pseudomonas aeruginosa and Acinetobacter baumannii worldwide has led to a re-evaluation of the previously discarded antibiotic, colistin. Introduction of colistin (Xylistin/Xylistin Forte) in India has proved to be important as salvage therapy for otherwise untreatable infections. However dosage guidelines for the prodrug colistin methanesulfonate (CMS) are not scientifically based and have led to treatment failure and increased colistin resistance, none in Indian settings.
Cipla conducted a pharmacokinetic study of CMS in patients with nosocomial infections caused by MDRGNB.
Nosocomial infections caused by MDRGNB have a mounting prevalence, are life threatening and challenging to treat.
Colistin, a multicomponent polypeptide polymyxin E antibiotic, has retained activity against these organisms.
To determine single and multiple dose pharmacokinetics of colistin (Xylistin Forte, Cipla Ltd, India) in critically ill patients.
Twelve consecutive patients with ventilator associated pneumonia and culture proven MDRGNB were enrolled. Colistin was administered intravenously, as colistimethate sodium for a minimum of 8 days and a maximum of 14 days. Serum samples were collected on first day and on steady-state day at predose, 30, 40, 60, 120, 240, 360 and 480 minutes after infusion, one predose sample for first seven days and concentrations were measured. Clinical response, bacteriological response and adverse events were recorded.
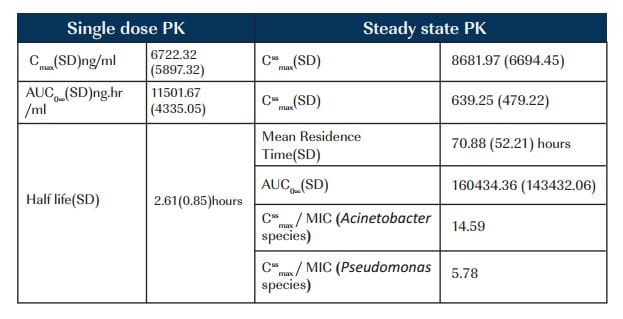
There were 7 males (median age 24.5 years) with an APACHE II score of 9.17; creatinine clearance of 118.09ml/min. MIC for Acinetobacter species was 0.595 mc^ml and Pseudomonas species was 1.5 mcg/ml.
Four patients had elevated liver enzymes (3 times ULN); one had hyponatremia, hypokalemia, and convulsions. Four patients died.
Patients responded adequately to the treatment with 67% survival rate. CssmaxMIC for Acinetobacter species was above optimal range of 8 to 10 for antibiotics having concentration dependant killing. Higher serum concentrations of colistin would be essential for MDR pseudomonal infections.