Overview of The Covid-19 Pandemic
Burden of the COVID-19 Pandemic [1-4]
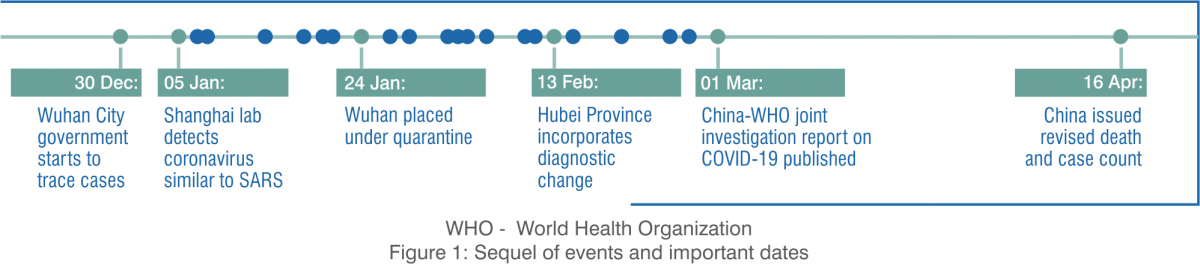
The current global pandemic, coronavirus disease 2019 (COVID-19) is due to the transmission of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). The outbreak of the new coronavirus infection, COVID-19 was initiated from the Hunan seafood market in Wuhan city in Hubei province in China in December 2019. The novel coronavirus is 30–60 times more lethal than the typical flu, with a current worldwide mortality rate of about 5.2%.
The disease poses enormous health, economic, environmental, political and social challenges to the entire human population. As far as the number of COVID-19 infected patients is concerned, as of June 2020 USA was at the top of the list followed by Brazil, Russia and India.
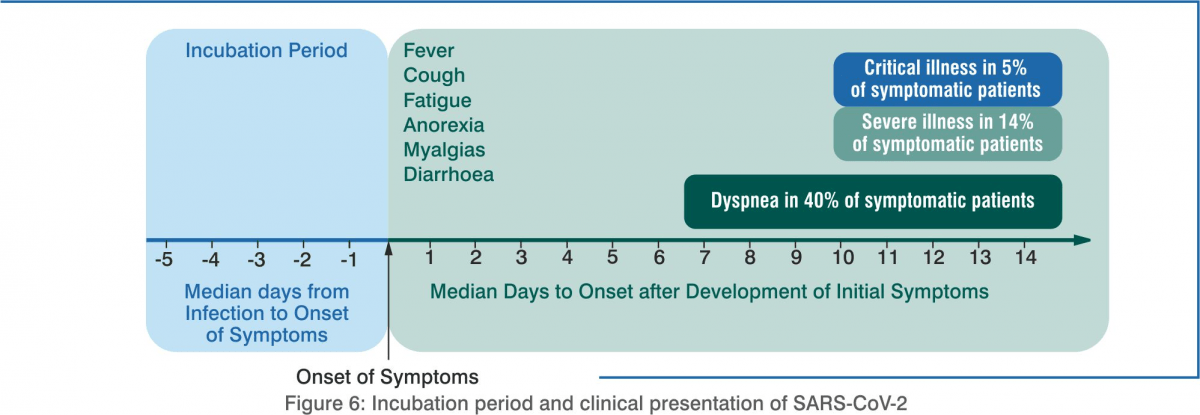
As of 23 June 2020, more than 9.29 million cases have been reported worldwide, resulting in 4,77,371 unfortunate deaths. The current recovery rate stands at 91% with more than 5 million recoveries. Mild symptoms include fever, dry cough, difficulty breathing, fatigue, and aches. Other symptoms are diarrhoea, sore throat, and loss of smell/taste. Post-exposure, it may take 5–14 days for symptoms to appear. Critical cases account for 5% of the total cases of COVID-19 is responsible for lower respiratory infection, may cause acute respiratory distress syndrome (ARDS). Older people or those with underlying health conditions are at a much higher risk of severe illness and mortality.
INDIA
The first case of coronavirus outbreak in India was reported on 30 January 2020 in Kerala's Thrissur district after a student had returned home from Wuhan University in China. As of 23rd July 2020, India had 12,08,773 confirmed cases out of which 7,82,606 recovered. The mortality rate in our country is down to 2.47% from the 3.38% in April. India has 30.04 cases per 100,000 population.
SARS-CoV-2[5,6]
COVID-19 shares similar characteristics with severe acute respiratory syndrome coronavirus (SARS-CoV); hence, formally, it is also known as SARS-CoV-2.
Structural Information
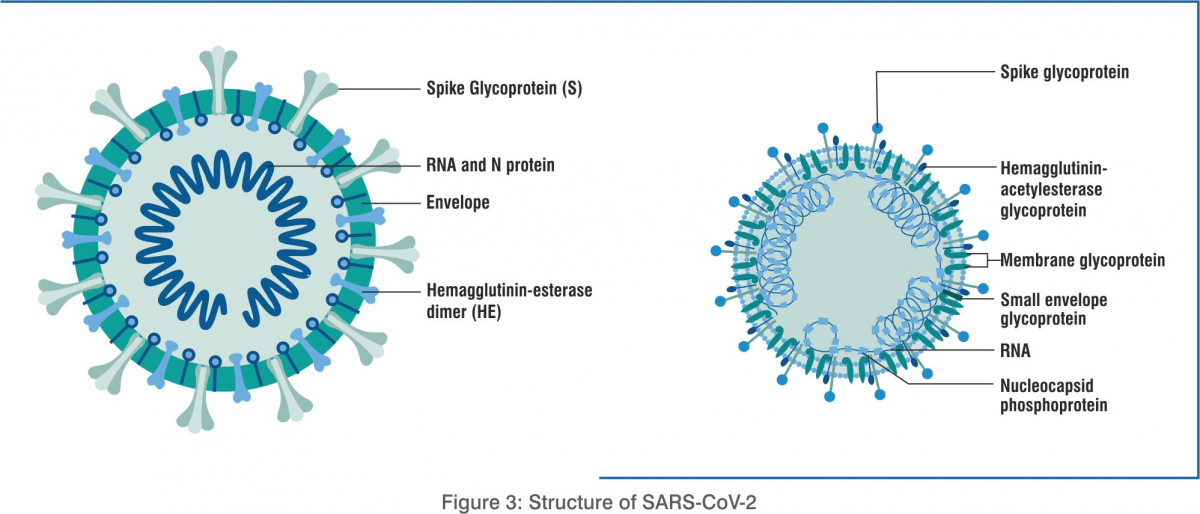
SARS-CoV-2 is an enveloped positive ssRNA coronavirus. Under a microscope, it has a crown-like appearance. The first step for virus entry into the human body occurs when the spike glycoprotein of SARS-CoV-2 on the cell surface binds to the angiotensin-converting enzyme 2 (ACE2) receptors of the host. Other proteins assist in virus pathogenesis and interfere with the host immune response. The viral membrane protein (M2) channel helps in membrane fusion due to an irreversible conformational change in the haemagglutinin (HA ). This facilitates the release of viral ribonucleoproteins (vRNPs) into the cytoplasm and nucleus and stimulates the transcription process. Neuraminidase (NA) is an enzyme responsible for cleaving sialic acid from the cell’s surface, permitting the new virion to be released from the cell and helping the virus transport in the bronchial cells.
Entry into the Host Cell: ACE2 Receptor
Analysis of the receptor-binding motif (RBM) in the spike glycoprotein showed that most of the amino acid residues essential for receptor binding were conserved between SARS-CoV and SARS-CoV-2, suggesting that the two CoV strains use the same host receptor for cell entry. The entry receptor utilised is ACE2. SARS-CoV-2 also binds to the ACE2 receptor after activation of the spike glycoprotein by the transmembrane protease serine 2 (TMPRSS2). Coronaviruses use the homotrimeric spike glycoprotein on the envelope to bind to their host cellular receptors. Such binding triggers a cascade of events that leads to a fusion between the cell and viral membranes, thereby enabling entry into the host cell, as depicted in Figure 4
Life cycle of SARS-CoV-2
Attachment: The spike glycoprotein of the virus binds to the ACE2 receptor of the host cell. Such binding triggers a sequence of events that cause the cell and viral membranes to fuse together, resulting in entry of the virus inside the host cell.
Entry: Viral genomic material (RNA) enters the host cell and is released in the cytoplasm.
Replication: The virus duplicates the genetic material along with other accessory proteins by a complex process that involves translation (protein synthesis). The enzyme responsible for replication of SARS-CoV-2 is RNA-dependent RNA polymerase (RdRp).
Assembly: Different parts of the virus, including capsids, are assembled.
Release: New virions are released from the infected cell.
Viral Shedding
Live coronavirus sheds at high concentrations from the nasal cavity even before symptom development. Such people are infectious and could transmit the virus unknowingly, indicating that there is a high level of SARS-CoV-2 shedding in the upper respiratory tract, even among pre-symptomatic patients. Viral load peaks approximately 10 days after symptom onset and could be detected up to 37 days in some patients.
Immune Response [7-13]
Host Immune Response
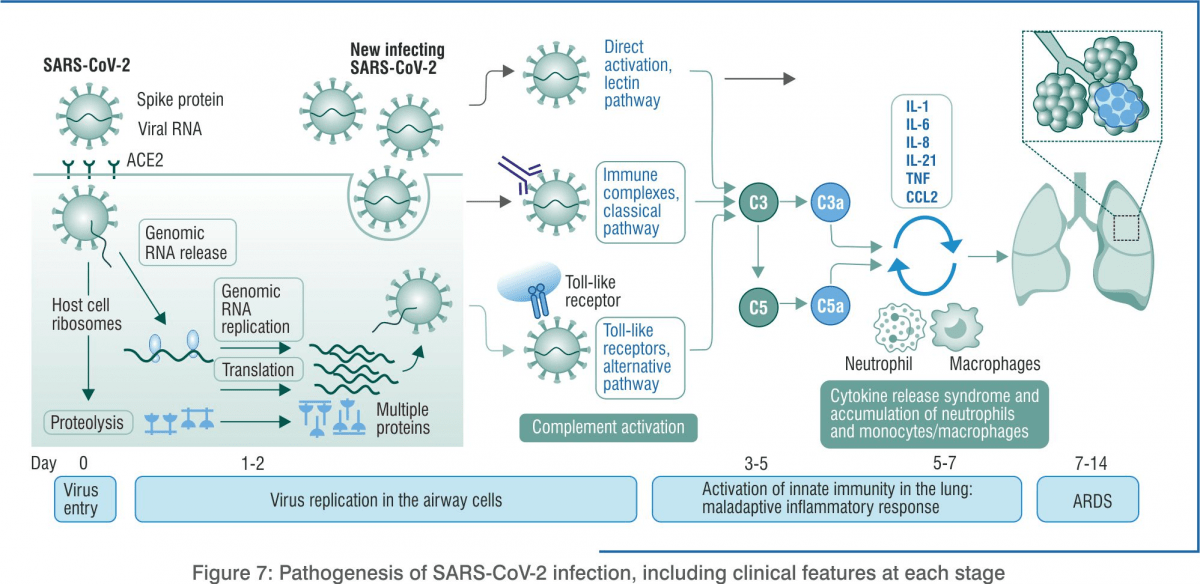
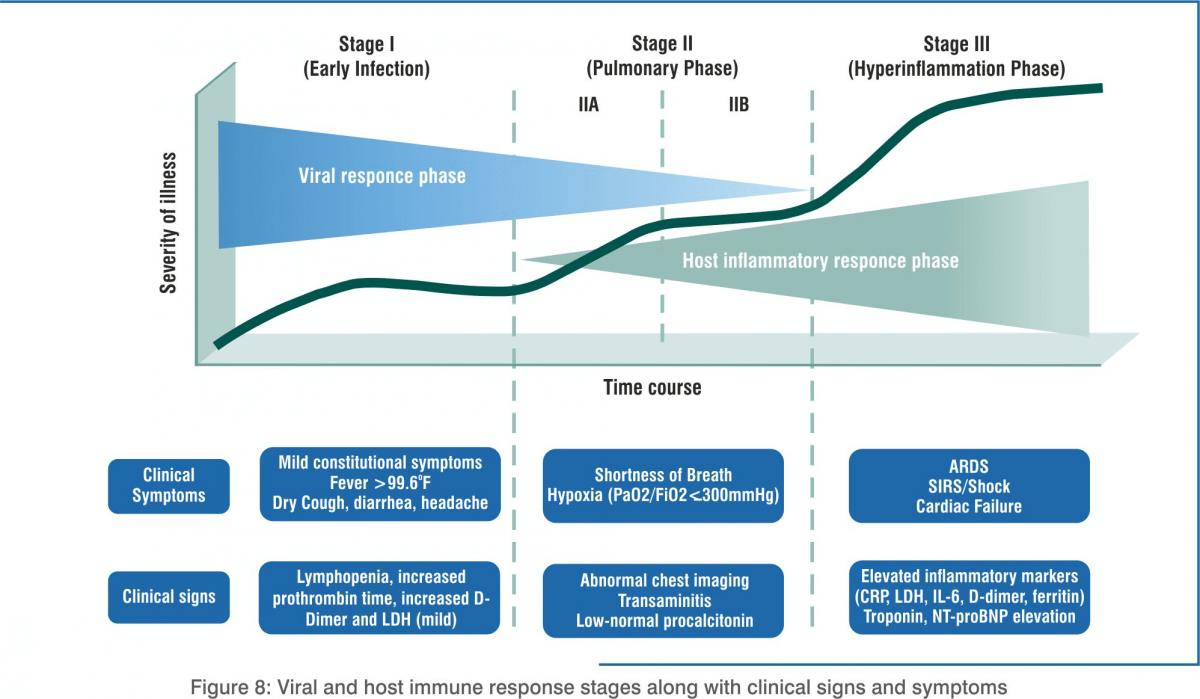
In stage 1, the body activates the immune system to trigger innate immunity while the virus has started replicating. Patients can be asymptomatic or pre-symptomatic. They may not have any symptoms for 1–2 days after being infected. Although the viral count may be low, these individuals are infectious in this phase, i.e. they can infect others by direct or indirect transmission.
Innate Immunity
When SARS-CoV-2 enters the human respiratory system, primarily through the alveolar macrophages type II cells, antigens are detected. The concentration of viral RNA increases as time proceeds. These virions are expelled by exocytosis from alveolar macrophage type II cells. In response to the infection, there is a significant increase in mRNA concentrations of interferon-beta, interferon-lambda (IL-29) and various pro-inflammatory cytokines and chemokines.
Adaptive Immunity – Humoural and Cellular
Latest reports show that the number of CD4+ and CD8+ T-cells in the peripheral blood of SARS-CoV-2-infected patients is significantly reduced. Overactivation of T-cells manifested by increase of T-helper cells (Th17) and high cytotoxicity of CD8 T-cells, accounts for, in part, the severe immune injury in severe conditions. B-lymphocytes produce antibodies that exacerbate organ damage via antibody-dependent enhancement (ADE). Similar to common acute viral infections, the antibody profile against SARS-CoV-2 virus has a typical pattern of immunoglobulin (IgM and IgG) production. The IgG antibody can last for a long time, which indicates that the IgG antibody may mainly play a protective role.
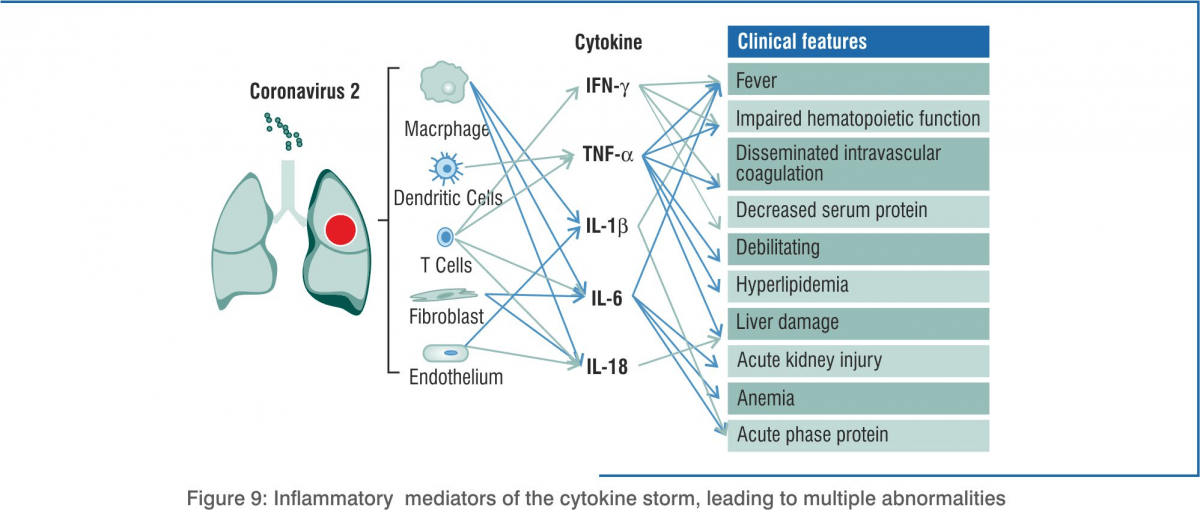
Cytokine Storm in COVID-19
The main cause of death due to COVID-19 is acute respiratory distress syndrome (ARDS). It also occurs in other coronavirus infections. Immune cells release a burst of cytokines and chemokines that are pro-inflammatory mediators, leading to an uncontrolled, systemic, deadly inflammatory response known as a ‘cytokine storm’. The immune system starts attacking the body. Patients with severe COVID-19 show elevated levels of serum IL-2, IL-7, IL-10, GCSF, IP-10, MCP-1, MIP-1alpha, TNF-alpha, IL-6, IFN-alpha, CCL-5, CXCL-8, and CXCL-10. Disease severity in patients is due to not only the viral infection but also the host response.
Symptoms, Diagnosis and Management
Symptoms [14,15]
In almost 80% of the cases of COVID-19, the patients tend to exhibit either nil or mild symptoms. However, almost 5% of the patients go on to develop a severe disease, which may eventually lead to death.
The presenting signs and symptoms of COVID-19 vary, as given below:
- Fever (83–99%)
- Cough (59–82%)
- Fatigue (44–70%)
- Anorexia (40–84%)
- Shortness of breath (31–40%)
- Myalgias (11–35%).
- Other non-specific symptoms, such as sore throat, nasal congestion, headache, diarrhoea, nausea and vomiting.
- Loss of smell (anosmia) or loss of taste (ageusia) preceding the onset of respiratory symptoms has also been reported.
- Older people and immunosuppressed patients in particular may present with atypical symptoms such as fatigue, reduced alertness, reduced mobility, diarrhoea, loss of appetite, delirium, and absence of fever.
- Symptoms such as dyspnoea, fever, gastrointestinal (GI) symptoms or fatigue due to physiologic adaptations in pregnant women, adverse pregnancy events, or other diseases such as malaria, may overlap with symptoms of COVID-19.
- In children, fever or cough has not been reported as frequently as in adults.
Risk Factors for Severe Disease
Older age and underlying conditions (see box below) increase the risk for severe infection. Elderly individuals are particularly at risk because of their diminished immune response and reduced ability to repair the damaged epithelium, reduced mucociliary clearance, and this may allow the virus to spread to the gas exchange units of the lungs more readily.
Underlying non-communicable diseases (NCDs)
Diagnosis [16,17]
Based on the presenting symptoms, a patient is advised to undergo the following tests to make a confirmatory diagnosis of COVID-19.
- Microbial tests
- Nucleic acid testing: Reverse transcription polymerase chain reaction (RT-PCR)
- Protein testing: Enzyme-linked immunosorbent assay (ELISA)
- Radiology: Chest X-ray, computed tomography (CT)
- Other blood tests
- Nucleic acid detection of a virus is done by a RT-PCR test. A swab specimen of the upper respiratory tract (URT) is collected by the nasopharyngeal and oropharyngeal route and sent for testing. In response to SARS-CoV-2, the immune system produces viral protein antigens and antibodies. These antigens and antibodies are detected by serological tests, which help to identify both prior and current infection and aid in proper diagnosis. These tests have less utility for diagnosis in the acute setting since antibodies are produced after several days to weeks of infection.
The Government of India recommends real-time or conventional RT-PCR tests for diagnosing COVID-19.
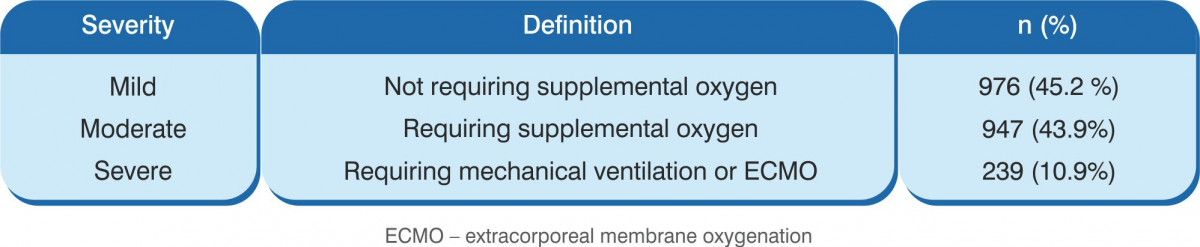
COVID-19 Disease Severity
The severity of COVID-19 is based on the clinical condition of the patient:
For detailed Classification please refer to Ministry of Health and Family Welfare, Government of India website
Management of COVID-19
Till date, there is no approved vaccine for COVID-19. All possible vaccines are undergoing clinical trials. Taking preventive measures and being cautious is the only option to control the spread of the virus. Control measures focus on reducing the R0 value to less than 1. (R0 is the average number of secondary infections produced by an infectious case in a population where everyone is susceptible and it is used to measure the transmission potential of a communicable disease).The World Health Organization (WHO) and other organisations have made the following general recommendations:
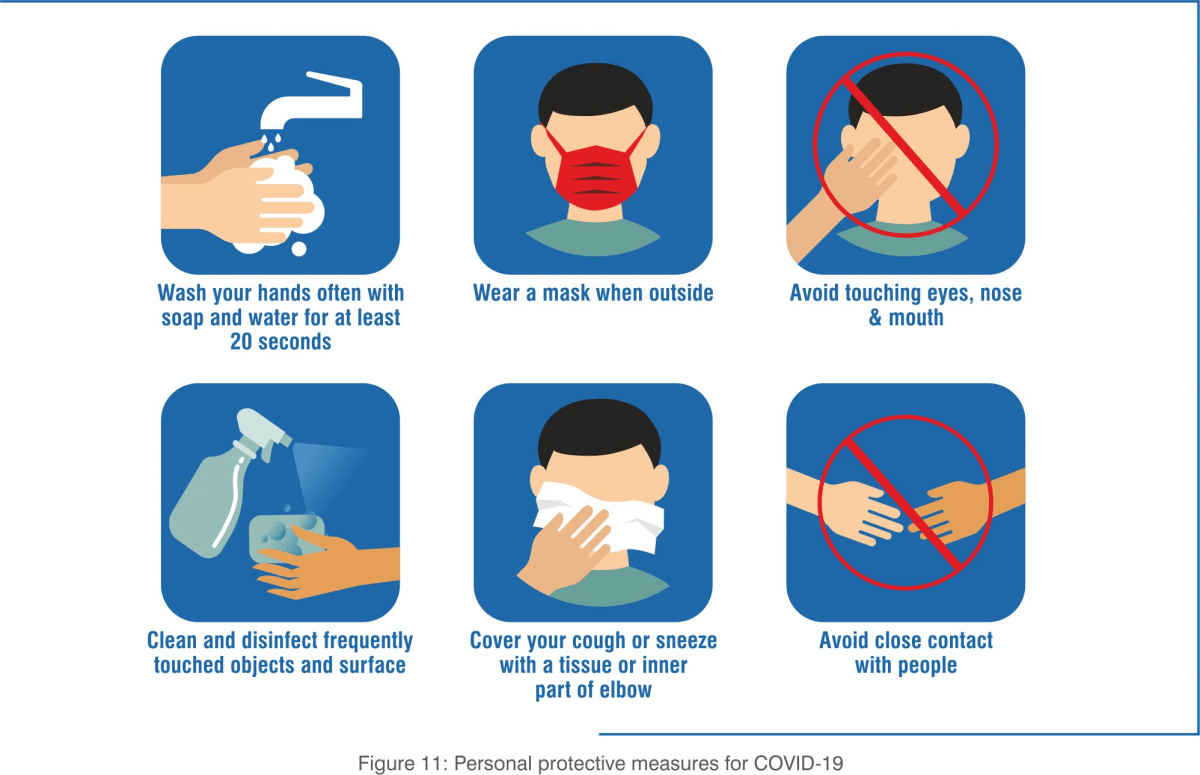
Personal Protective Measures
Clinical Management Protocol for COVID-19 by the Ministry of Health & Family Welfare (MoHFW), Government of India
- Mild cases: Can be managed at home. Offer symptomatic treatment such as antipyretic (paracetamol should be prescribed) for fever and pain, along with adequate nutrition and appropriate rehydration. Tab Hydroxychloroquine (HCQ) may be considered for any of those having high risk features for severe disease, under strict medical supervision
- Moderate cases: These cases usually require hospitalisation. Antibiotics should not be prescribed routinely without clinical suspicion of secondary bacterial infection. Provide oxygen support and prophylactic anticoagulation. Antivirals, Corticosteroids should be considered.
- Severe cases: These require early supportive care and monitoring, similar to measures taken in other critical cases.
For detailed management protocol please refer to Ministry of Health and Family Welfare, Government of India website)
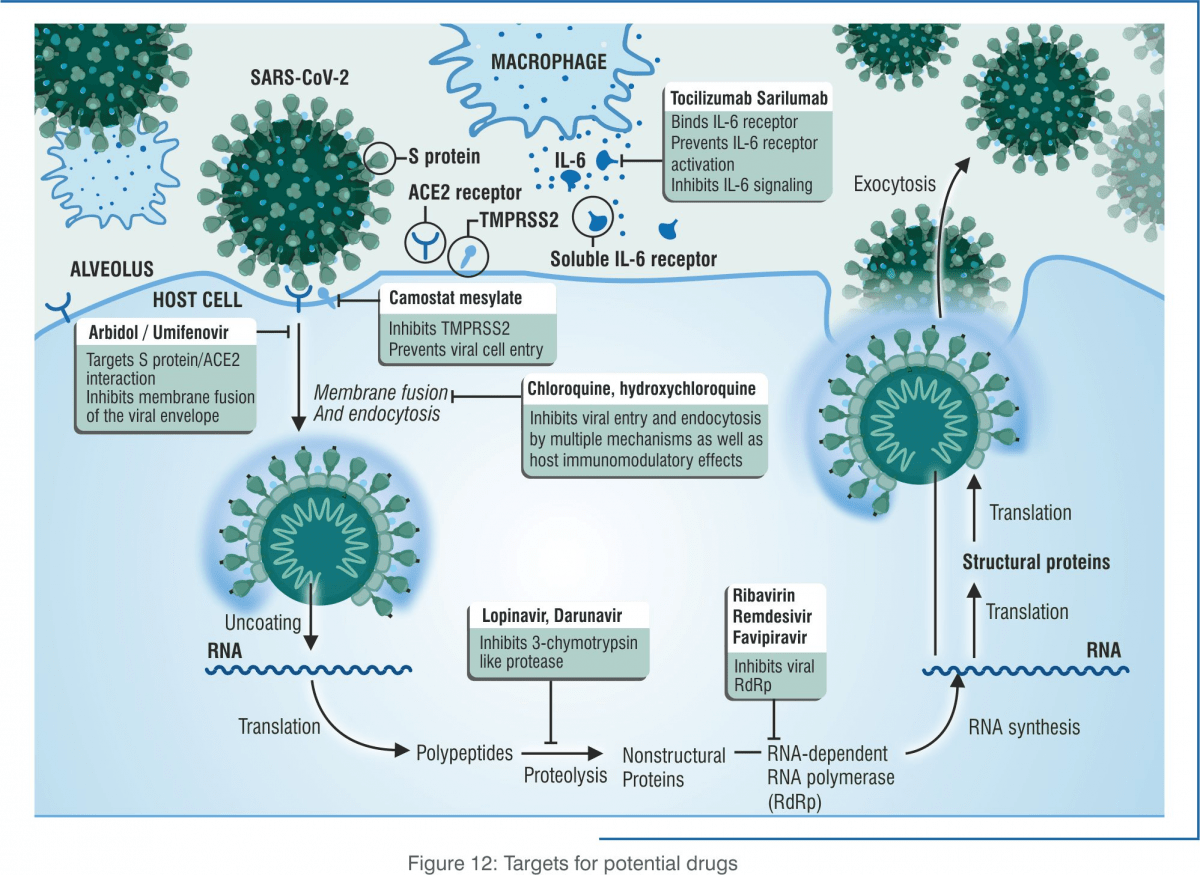
Researchers all around the world are testing the safety and efficacy of possible treatment options. Some drugs under clinical trial are known, i.e. they have previously been used for a different purpose. Such re-purposed drugs are being assessed to be efficacious in treating COVID-19.
Antiviral drugs such as remdesivir and favipiravir are two such repurposed antiviral drugs that have shown promising results in treating patients with COVID-19. On this basis, the Central Drugs Standard Control Organisation (CDSCO), India, has granted them Restricted Emergency Use Authorization during the pandemic.
Antiviral drugs: Restricted Emergency Use by the CDSCO, India
1. Remdesivir: injectable formulation for severe COVID-19 infection
2. Favipiravir: tablets for mild to moderate COVID-19 infection
About Favipiravir
Favipiravir
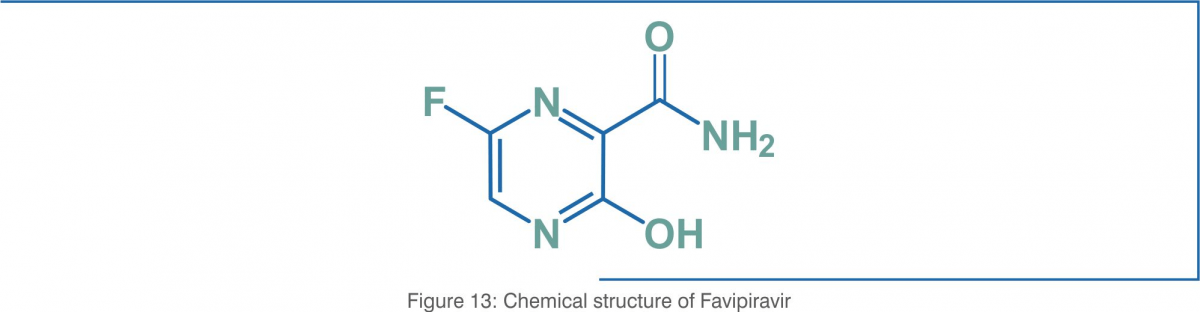
Favipiravir (T-705) (6-fluoro-3-hydroxypyrazine-2-carboxamide), a purine nucleic acid analogue, is a small molecule pyrazinecarboxamide derivative with activity against RNA viruses. Favipiravir was initially approved in 2014 in Japan for therapeutic use in resistant cases of influenza. The structural formula of favipiravir (molecular formula: C5H4FN3O2) is as below:
Favipiravir has shown promise in the treatment of avian influenza, and may be an alternative option for influenza strains that are resistant to neuramidase inhibitors. Favipiravir has been investigated for the treatment of life-threatening pathogens such as Ebola virus, Lassa virus, and has now been authorised for restricted use in COVID-19.
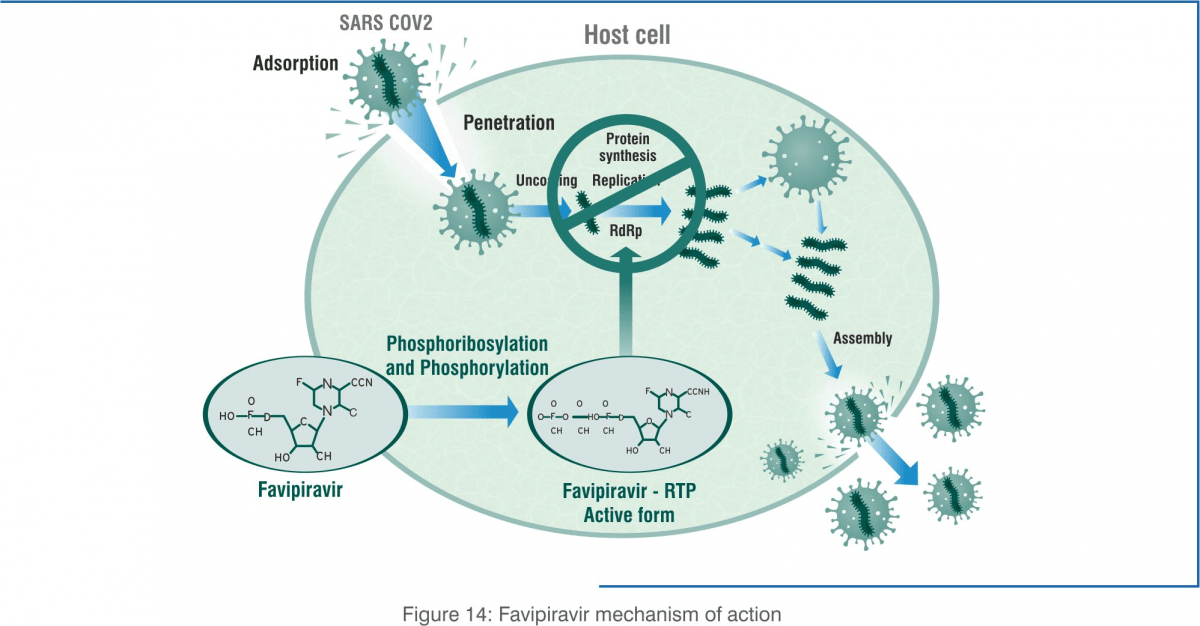
Mechanism of Action [20,21]
Favipiravir (prodrug) undergoes intracellular metabolism to favipiravir ibofuranosyl-5'- triphosphate (T-705RTP). The active metabolite (T-705RTP) inhibits RNA-dependent RNA polymerase (RdRp) enzymes, which are necessary for replication of the viral genome.
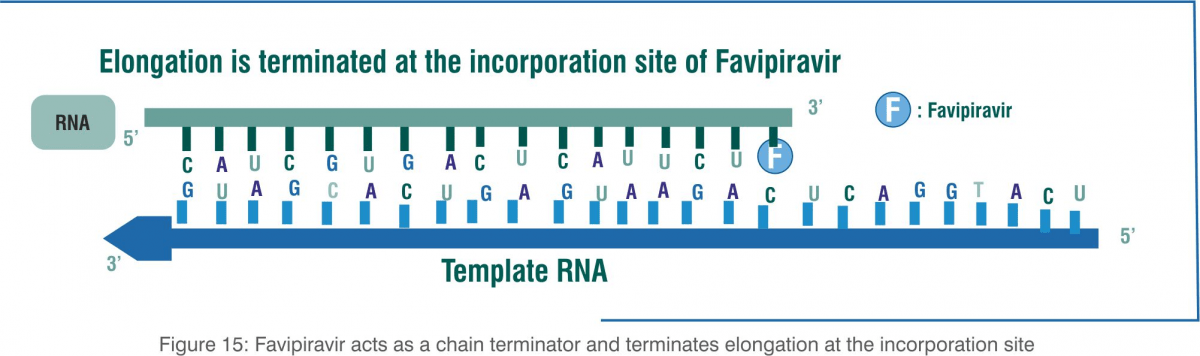
Favipiravir functions as a purine analogue, and it is incorporated instead of guanosine and adenosine. It terminates elongation after the incorporation of a single favipiravir molecule instead of guanosine and adenosine. After the incorporation of two consecutive favipiravir molecules, the synthesis of this complimentary viral RNA strand cannot be completed.
The incorporation of favipiravir into the viral genome terminates elongation, resulting in shorter genome sizes and the marked loss of the viral genome in favipiravir-treated cultures. Viral RdRp lacks proofreading activity and is unable to complete the elongation step when favipiravir is incorporated as a chain terminator.
In vitro Activity of Favipiravir against SARS-CoV-2[24]
Method:
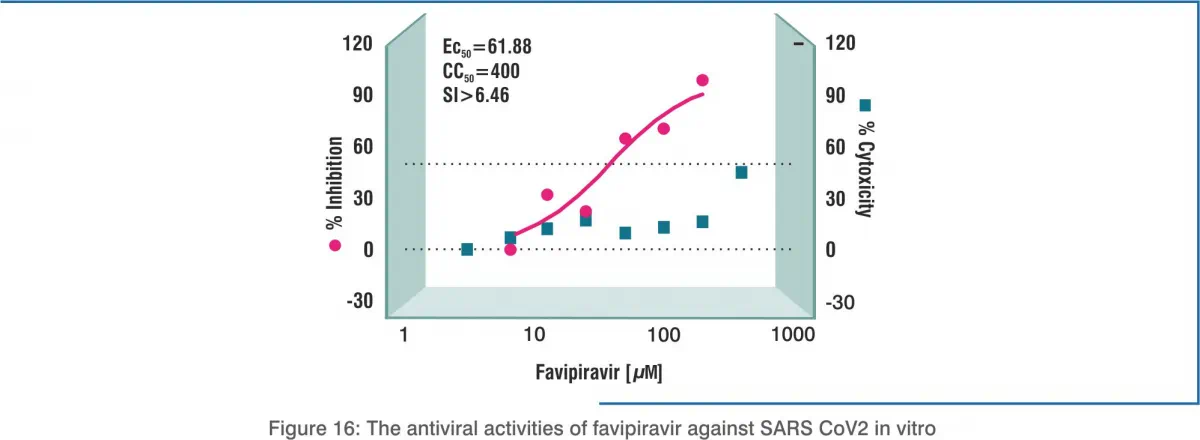
Standard assays were carried out to measure the effects of favipiravir on the cytotoxicity, virus yield and infection rates of SARS- CoV2. Dimethyl sulphoxide (DMSO) was used as the control. Cytotoxicity of the candidate compounds in Vero E6 cells was determined by the cell counting kit-8 (CCK8) assay. Vero E6 cells were infected with nCoV-2019BetaCoV/Wuhan/WIV04/20192 at a multiplicity of infection (MOI) of 0.05 in the presence of varying concentrations of the test drugs. Efficacy was evaluated by quantification of viral copy numbers in the cell supernatant via quantitative real-time RT-PCR (qRT-PCR). Confirmation with visualisation of virus nucleoprotein (NP) expression through immunofluorescence microscopy at 48 hours post-infection.
Result:
In vitro, the 50% effective concentration (EC50) of favipiravir against SARS-CoV-2 was 61.88 μM/L in Vero E6 cells, indicating that dose concentrations higher than the dose used in influenza may be required. Further, half-cytotoxic concentration (CC50) of more than 400 μM and a selectivity index (SI) of more than 6.46 were required to reduce the viral infection.
Pharmacokinetics [22,23]
Absorption
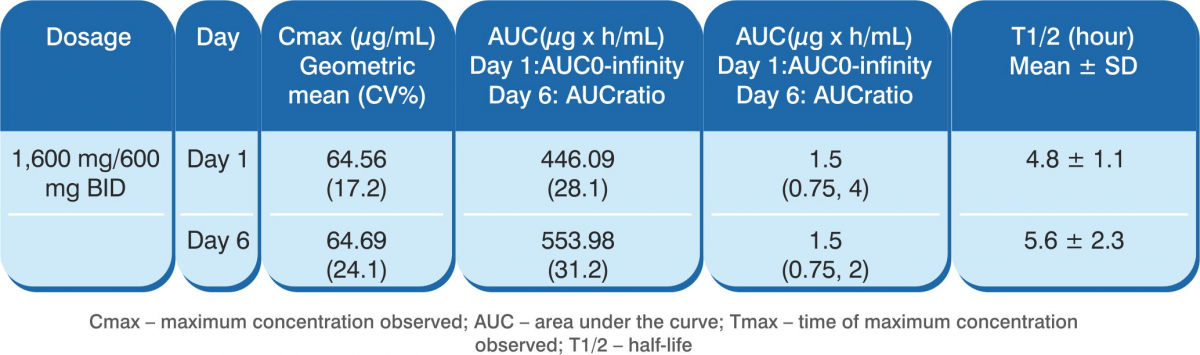
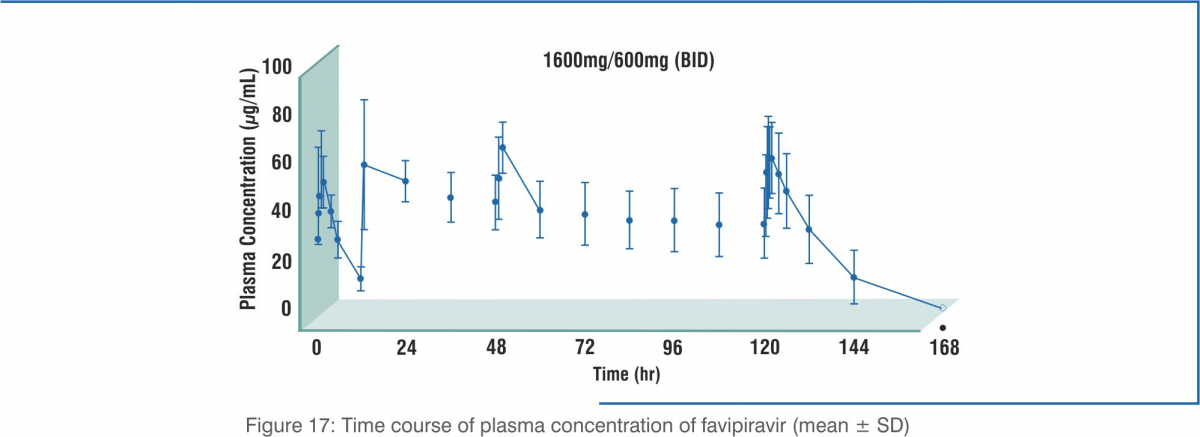
The following table shows the pharmacokinetic parameters of favipiravir after oral administration in 8 healthy adults at 1,600 mg twice daily for 1 day, and then 600 mg twice daily for 4 days followed by 600 mg once daily for 1 day (1,600/600 mg BID).
Following multiple oral administration of favipiravir (1,200 mg + 400 mg on day 1, then 400 mg twice daily on days 2 to 6 followed by 400 mg once daily on day 7) for 7 days to healthy adults who appeared to have little aldehyde oxidase (AO) activity, the estimated area under the curve (AUC) of unchanged drug was 1,452.73 µ × hour/mL on day 1 and 1,324.09 73 µ × hour/mL on day 7.
Distribution
When favipiravir was orally administered to 20 healthy adult male subjects at 1,200 mg twice daily for 1 day followed by 800 mg twice daily for 4 days (1,200 mg/800 mg BID), the geometric mean concentration of the drug in semen was 18.341 µg/mL on day 3, and 0.053 µg/mL on the second day after the treatment. The semen levels went below the limit of quantification (0.02 µg/mL) in all subjects in 7 days after the end of the treatment. The mean ratio of the drug concentration in semen to that in plasma was 0.53 on day 3 and 0.45 on day 2 after the treatment. The serum protein-binding ratio was 53.4 to 54.4% (in vitro, centrifugal ultrafiltration) at 0.3 to 30 µg/mL.
Metabolism
Favipiravir is not metabolised by cytochrome P450 (CYP). It is mostly metabolised by aldehyde oxidase (AO), and partly metabolised to a hydroxylated form by xanthine oxidase (XO). In studies using human liver microsomes, formation of the hydroxylate ranged from 3.98 to 47.6 pmol/g protein/minute, with an inter-individual variation of AO activity by 12 times the maximum. A glucuronate conjugate was observed other than the hydroxylated form.
Excretion
Favipiravir was mainly excreted as a hydroxylated form into the urine, and little amount unchanged drug was observed. In an oral 7-day multiple-dose study with 6 healthy adults, cumulative urinary excretion ratio of the unchanged drug and the hydroxylated form was 0.8% and 53.1%, respectively, during 48 hours after the last administration.
Note: The study was conducted using 1,200 mg + 400 mg on day 1, then 400 mg BID on days 2–6 followed by 400 mg OD on day 7.
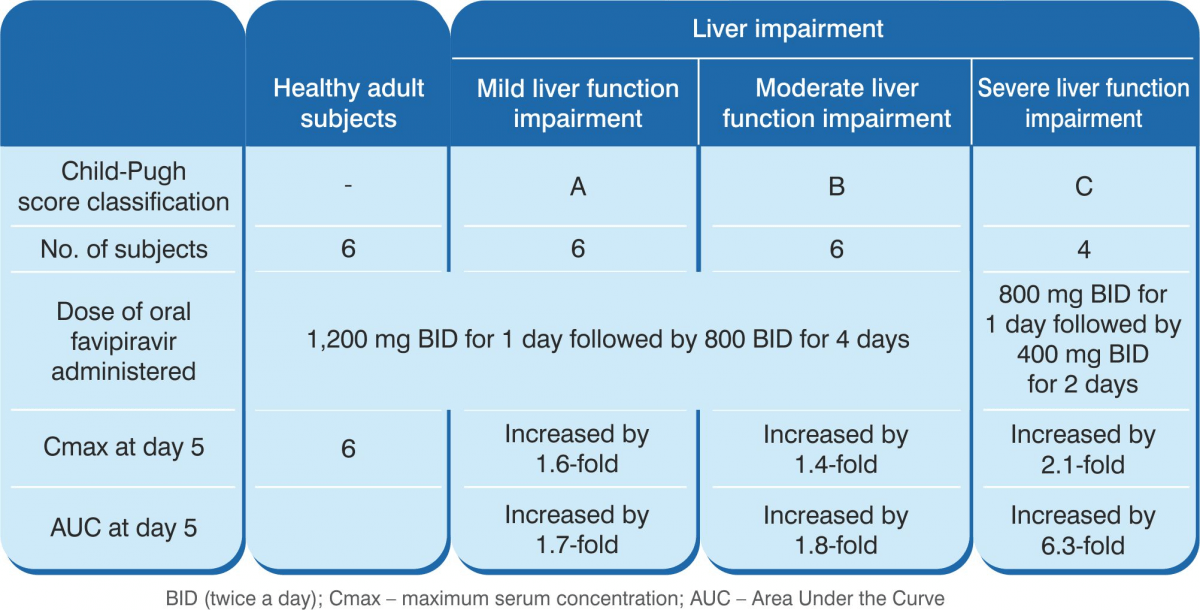
Mild-to-moderate hepatic impairment had a moderate impact on the pharmacokinetics of favipiravir. The pharmacokinetics of favipiravir was substantially altered in subjects with severe hepatic impairment (Child-Pugh class C) such that AUC values based on the total concentrations were 2.1-fold and 6.3-fold on day 1 and day 5 respectively, compared with those in subjects with normal hepatic function. As a result, favipiravir 1,200/800 mg BID is currently not recommended for COVID-19 patients with severe hepatic impairment (Child-Pugh class C).
Drug Interactions
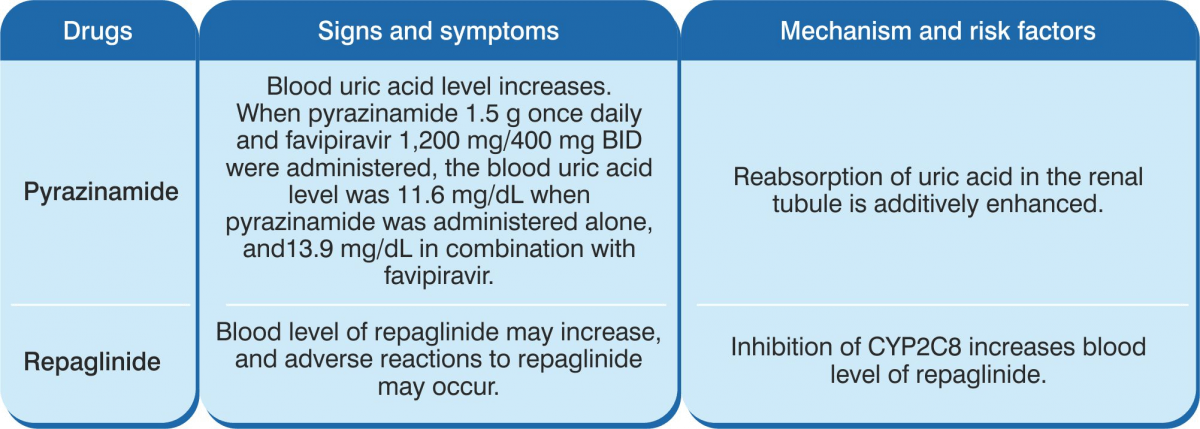
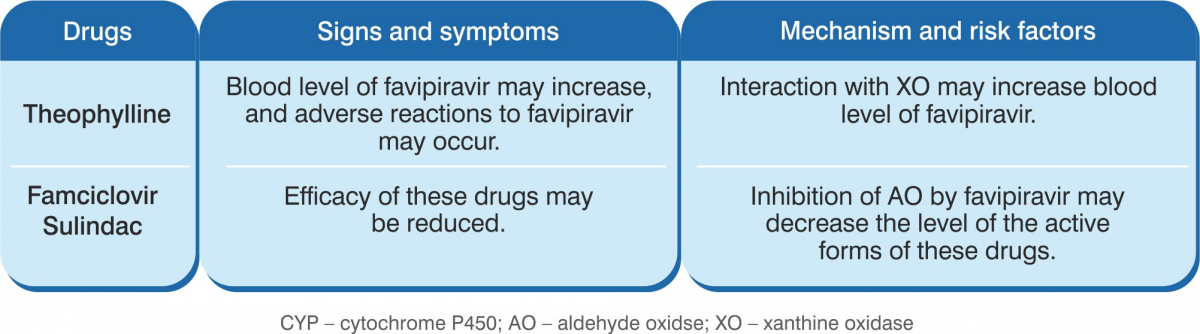
Favipiravir is not metabolised by CYP450. It is mostly metabolised by aldehyde oxidaase (AO), and partly metabolised by XO. The drug inhibits AO and CYP2C8, but does not induce CYP.
Indications
Favipiravir is indicated for the treatment of patients with mild-to-moderate COVID-19.
Recommended Dose and Dosage Adjustment
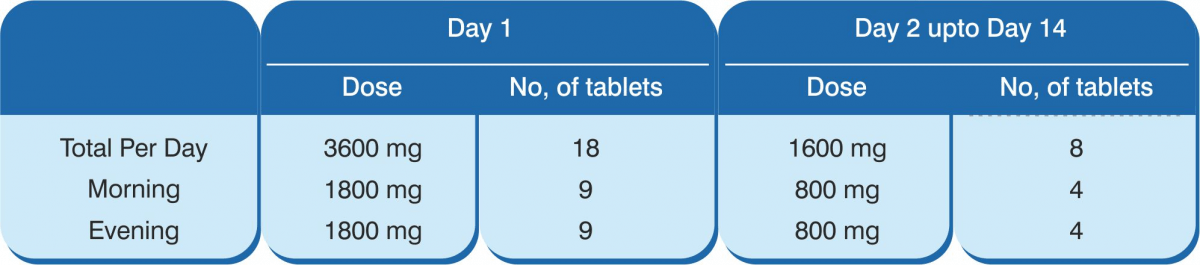
1,800 mg orally twice daily on day 1, followed by 800 mg twice daily up to a maximum of 14 days.
The administration should be started promptly after suspected or laboratory confirmation of SARS CoV-2 infection in adults with mild to moderate disease.
Use in Elderly: Since the elderly often have reduce physiological function, favipiravir should be administered with care to them by monitoring their general condition
Use in Children: Favipiravir has not been administered to children
Clinical Evidence on Favipiravir in COVID-19
Experimental Treatment with Favipiravir for COVID-19
An Open-Label Controlled Study [25]
 Study design:
Open-label, non-randomised, before-after controlled study.
Study design:
Open-label, non-randomised, before-after controlled study.
 Eligibility criteria: Patients, 16–75 years of
age, who tested positive for the novel coronavirus by nasopharyngeal swab samples; and, duration from disease onset
to study enrolment was less than 7 days.
Eligibility criteria: Patients, 16–75 years of
age, who tested positive for the novel coronavirus by nasopharyngeal swab samples; and, duration from disease onset
to study enrolment was less than 7 days.
Patients with severe clinical conditions such as respiratory failure, shock, or organ failure requiring intensive care unit (ICU) monitoring were excluded.
 Treatment
duration: 14 days or until viral clearance was confirmed.
Treatment
duration: 14 days or until viral clearance was confirmed.
Time of viral clearance
Improvement rate of CT scans on day 14 after treatment
Safety assessment
Results
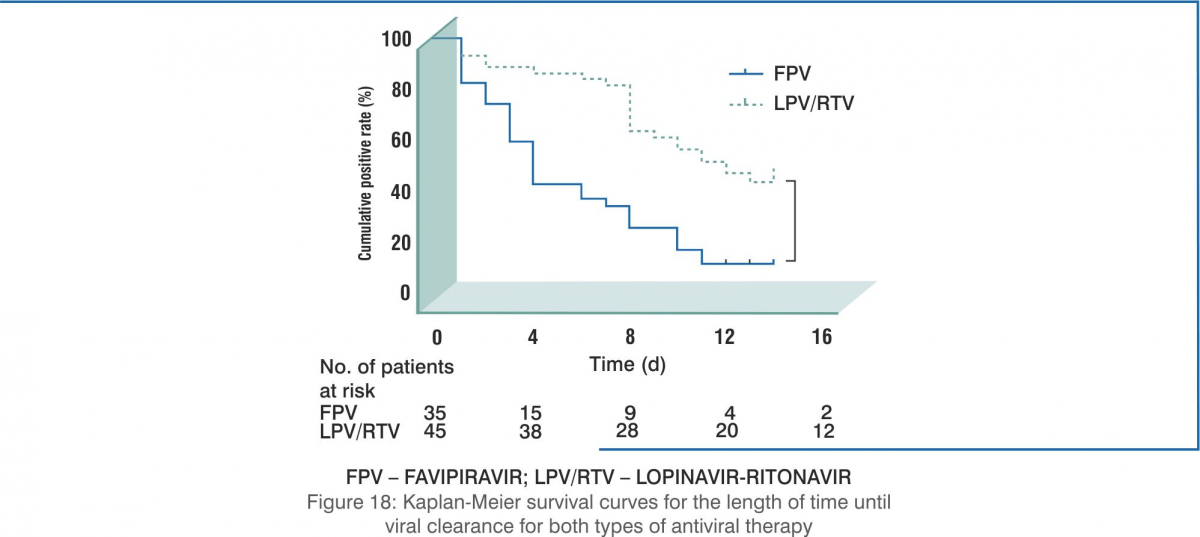
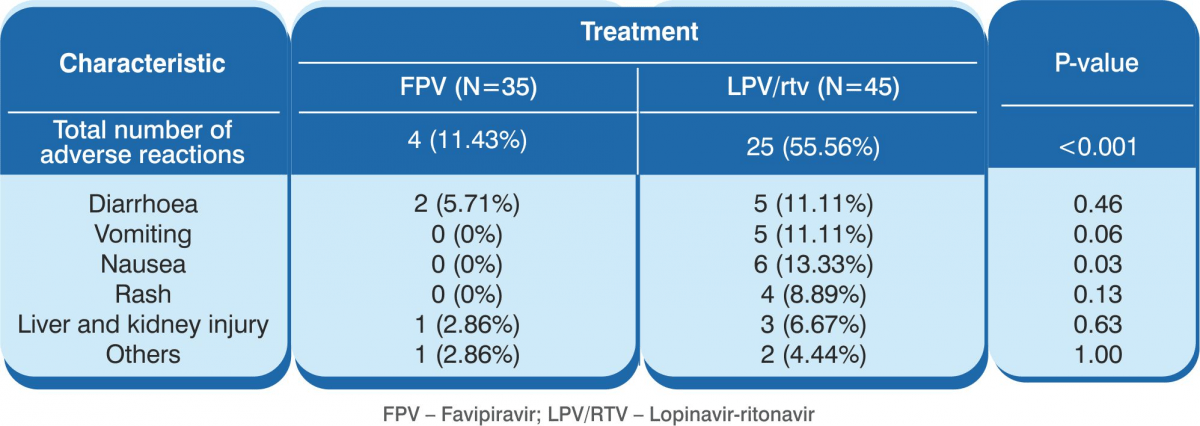
- The results showed that the 35 patients in the favipiravir arm demonstrated significantly shorter viral clearance time as compared with the 45 patients in the control arm (median, 4 days versus 11 days, P<0.001).
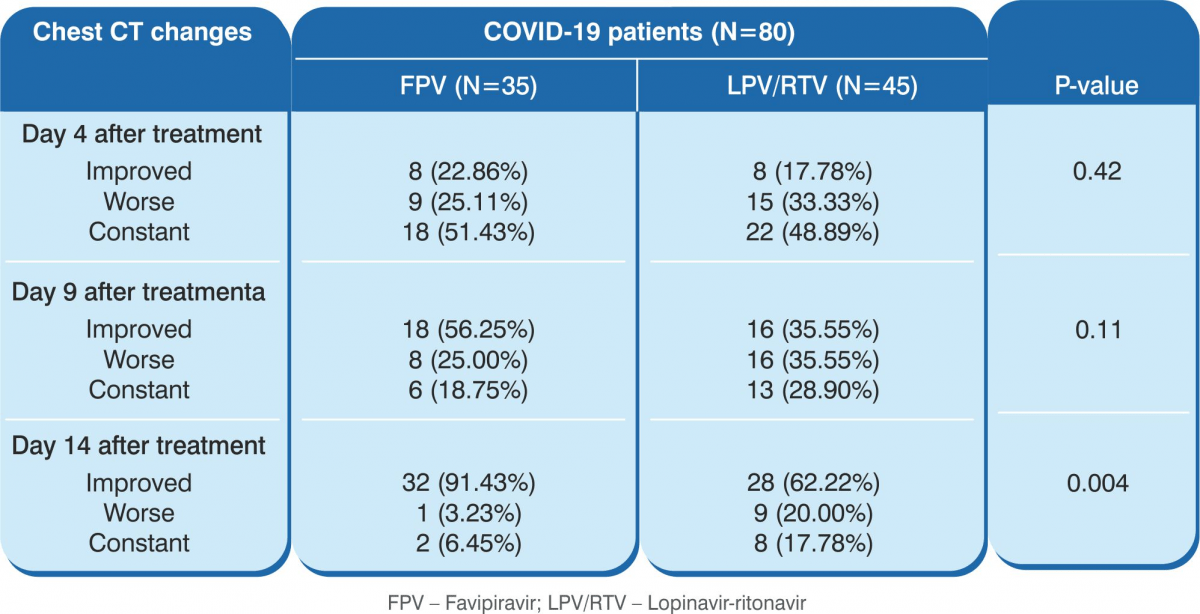
- CT scan confirmed a higher rate of improvement in the favipiravir arm (91.43% versus 62%, P=0.004).
- Significantly fewer adverse events were reported in the favipiravir arm than in the control arm. There were 4 (11.43%) adverse events in the favipiravir arm as against 25 adverse events (55.56%) in the control arm (P<0.001)
Conclusion: Favipiravir showed better treatment outcomes in COVID-19 patients in terms of their disease progression and viral clearance. Favipiravir was also found to be safe and well tolerated.
Favipiravir versus Umifenovir for COVID-19
A Randomised Clinical Trial [26]
 Study design:
Prospective, randomised, controlled, open-label, multicentre trial.
Study design:
Prospective, randomised, controlled, open-label, multicentre trial.
 Eligibility
criteria: Patients aged 18 years or older diagnosed with COVID-19 pneumonia and having initial symptoms
within 12 days were enrolled in the study. Diagnostic criteria included (1) a positive chest CT scan; (2)
significant clinical manifestation, including pyrexia, cough, breathing difficulty and other indications of viral
infection of lower respiratory tract; and, (3) laboratory results indicating lymphopaenia and (optional)
leucopaenia. Severe/critical patients whose expected survival time was less than 48 hours were excluded.
Eligibility
criteria: Patients aged 18 years or older diagnosed with COVID-19 pneumonia and having initial symptoms
within 12 days were enrolled in the study. Diagnostic criteria included (1) a positive chest CT scan; (2)
significant clinical manifestation, including pyrexia, cough, breathing difficulty and other indications of viral
infection of lower respiratory tract; and, (3) laboratory results indicating lymphopaenia and (optional)
leucopaenia. Severe/critical patients whose expected survival time was less than 48 hours were excluded.
 Intervention:
Patients received either of the following:
Intervention:
Patients received either of the following:
- Favipiravir 1,600 mg BID on the first day followed by 600 mg BID for the following days
OR
- Umifenovir 200 mg TID plus standard care
 Treatment
duration: 7 days. The treatment could be extended to 10 days as per the judgement of the
researchers.
Treatment
duration: 7 days. The treatment could be extended to 10 days as per the judgement of the
researchers.
a) Clinical recovery rate at day 7. [Continuous (>72 hours) recovery of body temperature, respiratory rate, oxygen saturation and cough relief after treatment, with the following quantitative criteria: axillary temperature ≤ 36.6°C; respiratory frequency ≤ 24 times/minute; oxygen saturation ≥ 98% without oxygen inhalation; and, mild or no cough.]
a) The latency to pyrexia relief and the latency to cough relief
b) The rate of auxiliary oxygen therapy (AOT) or non-invasive mechanical ventilation (NMV)
c) All-cause mortality
d) Dyspnoea
e) Rate of respiratory failure (defined as SpO2 < 90% without oxygen inhalation or PaO2/FiO2)
f) Safety – adverse events that occurred and premature discontinuation of drug
Results
Primary outcomes
- The clinical recovery rate did not differ between the two groups. At day 7, 62/120 (51.67%) in the umifenovir group and 71/116 (61.21%) patients in the favipiravir group (P=0.1396) clinically recovered. However, in the moderate COVID-19 group, clinical recovery on day 7 was significantly higher in the favipiravir arm (71.43%) compared with the umifenovir arm (55.86%), P=0.0199.
Secondary outcomes
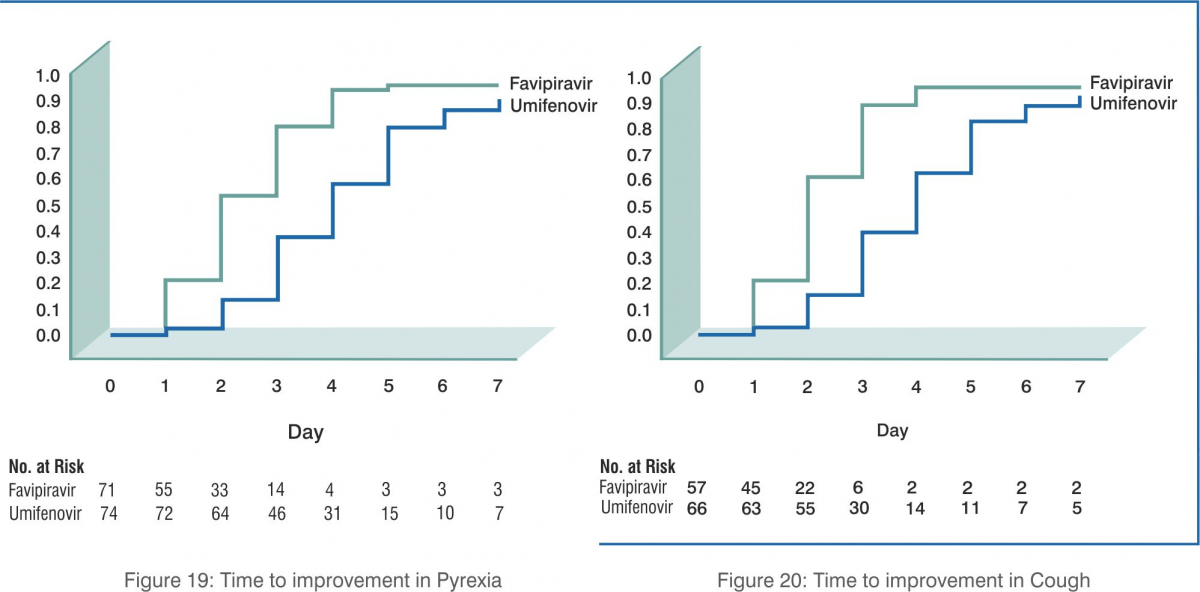
- Favipiravir led to shorter latencies to relief for both pyrexia and cough (p<0.0001).
- Favipiravir was not associated with a decreased rate for AOT or NMV, dyspnoea, overall respiratory failure rate, ICU admission or all-cause mortality.
- Incidence of dyspnoea during the treatment was lower in the favipiravir arm (p=0.0174).
- Safety: The most common adverse effect was raised serum uric acid levels (16/116); odds ratio: 5.52 (p<0.0014). No differences were observed in the ALT (Alanine transaminase) or AST (Aspartate transaminase) elevations.
Conclusion: Favipiravir is associated with significantly shortened latency to relief for pyrexia and cough. Antiviral-associated adverse effects of favipiravir are mild and manageable.
Observational Study on Favipiravir- Results from A Japanese Registry [27]
 Study
design: Observational study.
Study
design: Observational study.
 Methodology: In Japan, favipiravir is being provided
to hospitals admitting confirmed COVID-19 patients after a request for compassionate use was made to the Ministry of
Health, Labour and Welfare (MoHFW). The hospitals are asked at the time of such provision to join the Favipiravir
Observational Study and provide information regarding the patient demographics, comorbidities, severity of illness,
dose and duration of favipiravir, use of other medications targeting SARS-CoV-2, adverse events, clinical status at
7 and 14 days from the start of the use of favipiravir, and the clinical outcome approximately 1 month after
admission to the hospital.
Methodology: In Japan, favipiravir is being provided
to hospitals admitting confirmed COVID-19 patients after a request for compassionate use was made to the Ministry of
Health, Labour and Welfare (MoHFW). The hospitals are asked at the time of such provision to join the Favipiravir
Observational Study and provide information regarding the patient demographics, comorbidities, severity of illness,
dose and duration of favipiravir, use of other medications targeting SARS-CoV-2, adverse events, clinical status at
7 and 14 days from the start of the use of favipiravir, and the clinical outcome approximately 1 month after
admission to the hospital.
Results
- A total of 2,158 cases were registered from 407 hospitals till 15 May 2020.
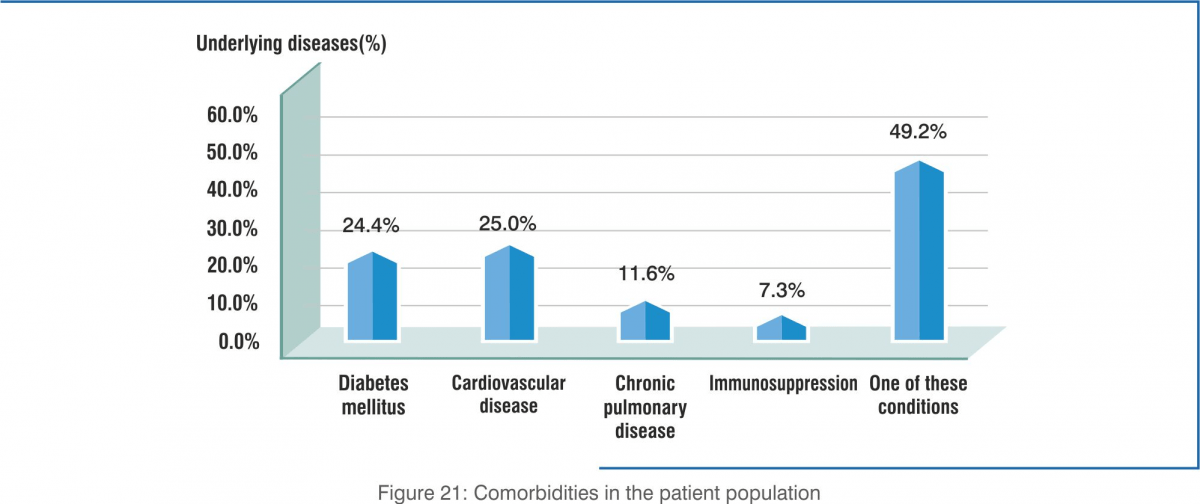
- Most of the patients (49.2%) had at least one comorbidity (diabetes, cardiovascular diseases, chronic lung diseases, immunosuppression) Underlying diseases were common, especially diabetes and cardiovascular disease.
- Concomitant antiviral medication prescribed – ciclesonide (41.6%) and lopinavir-ritonavir (3.4%).
- Baseline severity of disease – patients had mild to severe disease.
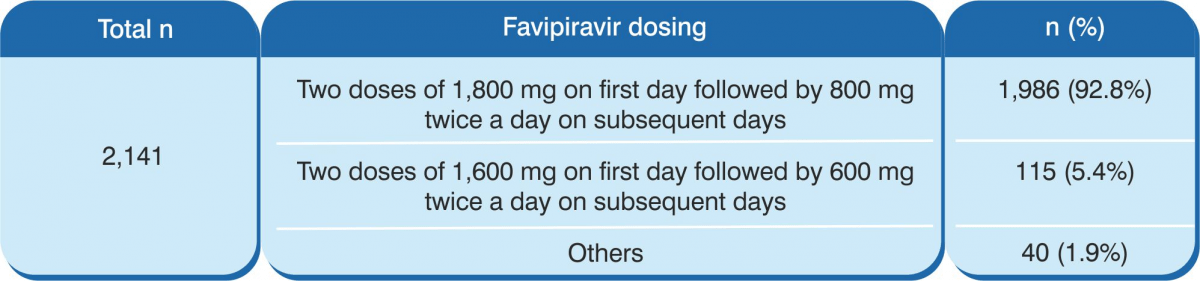
- A total of 2,141 patients received favipiravir.
- Administration of favipiravir – in 92.8% of the patients, favipiravir was administered as two doses of 1,800 mg orally on the first day followed by 800 mg orally twice a day on subsequent days. The median duration was 11 days. The median days from the positive PCR test and hospital admission to the initiation of favipiravir therapy were 2 days and 1 day, respectively.
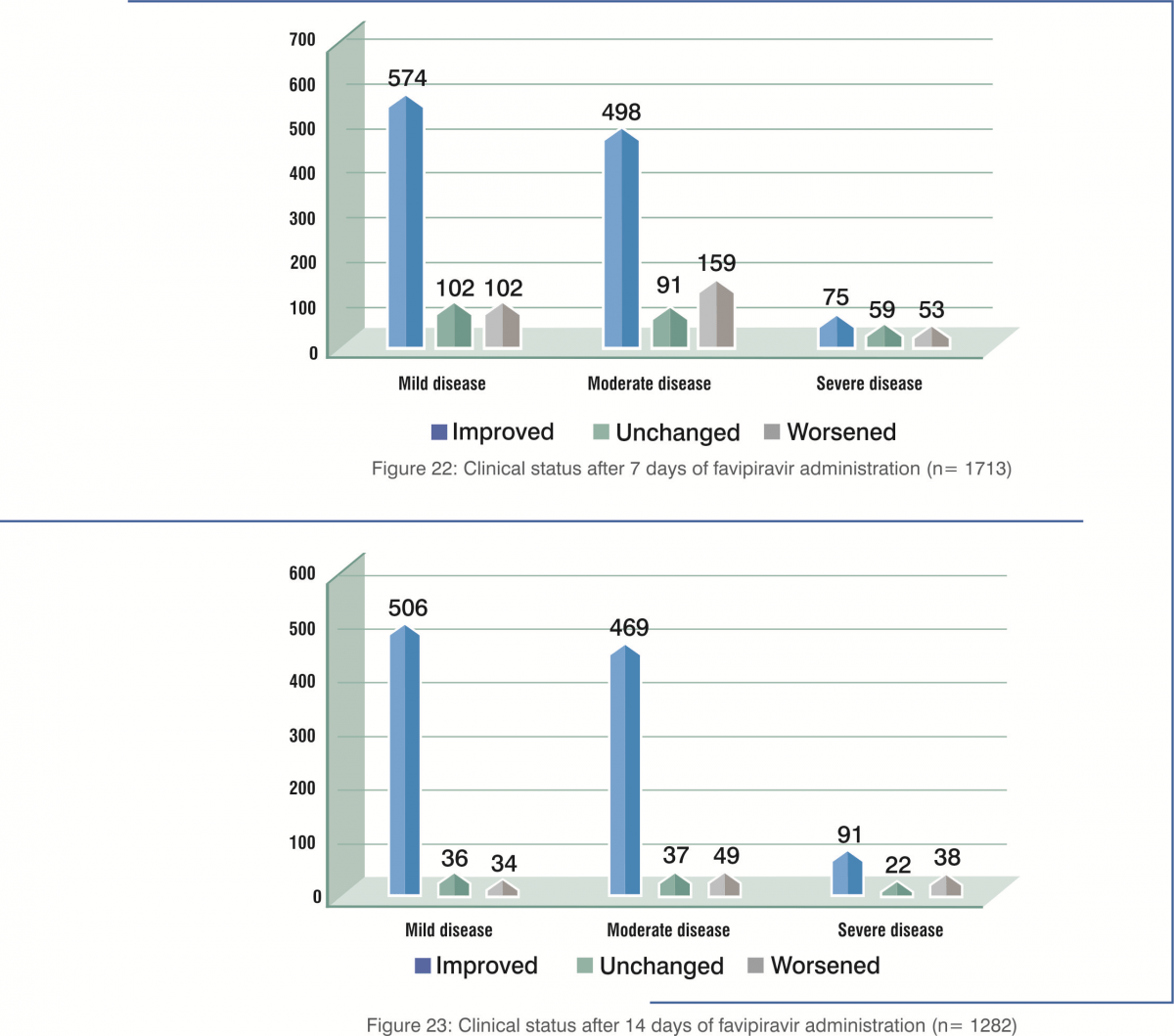
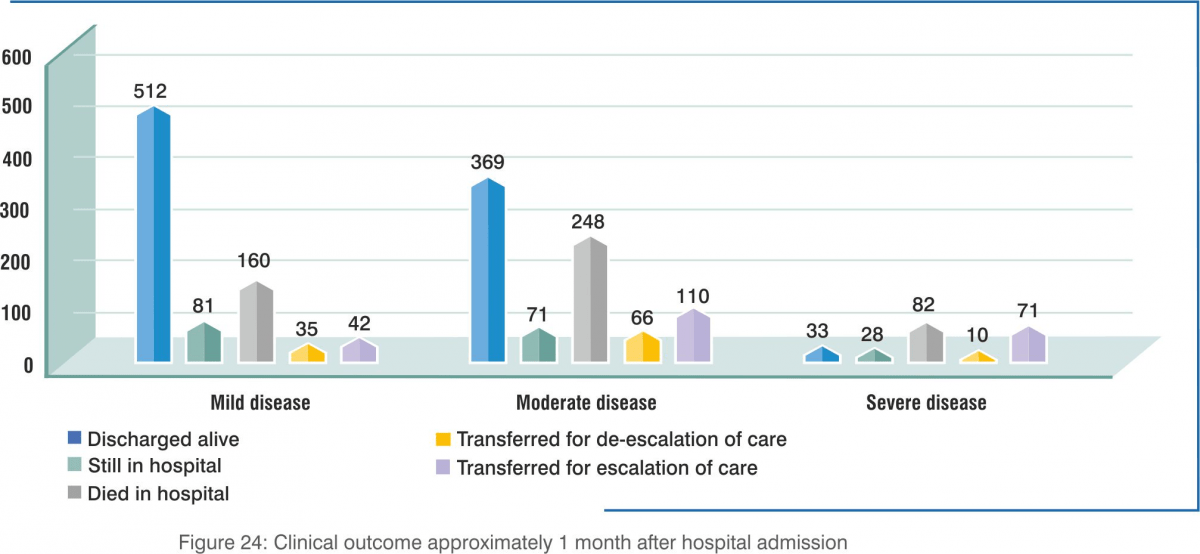
Patients with mild-to-moderate illness had better clinical improvement on day 7 and day 14.
- Clinical improvement rates were 79.0% at 7 days and 92.4% at 14 days for those less than 60 years old, whereas they were 55.0% at 7 days and 73.8% at 14 days for those 60 years of age or older.
- The mortality rates at the time of survey were 1.8% for those 59 years of age or younger, and 20.8% for those 60 years of age or older.
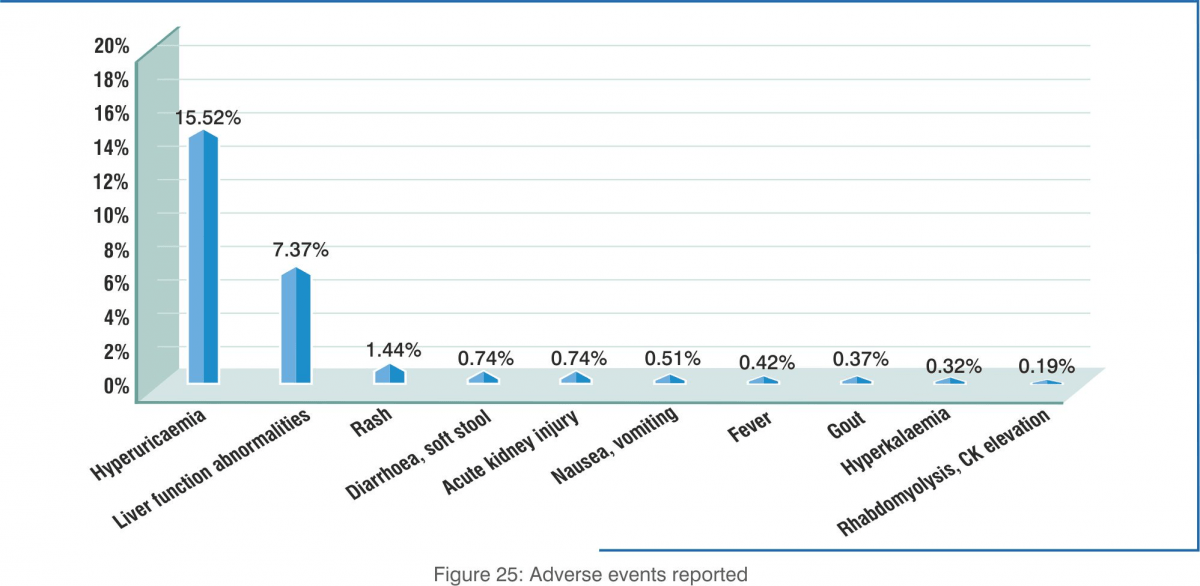
- Adverse events: A total of 626 events were reported in 532 patients. The most common were hyperuricaemia (335 patients; 15.52%) followed by liver injury or liver function test (LFT) abnormalities (159 patients; 7.37%). Both of these are known adverse events of favipiravir. No new trends were observed.
Conclusion:
- The data reported suggests that the vast majority of patients with mild and moderate illness recovered, whereas poor prognosis is not uncommon among those with severe illness.
- Hyperuricaemia and liver function abnormalities were the most commonly observed adverse events associated with favipiravir use, which was expected based on the known safety profile of this agent.
Open-Label, Comparative, Randomised Study[28]
Study design: A multicentre, randomised, open-label, comparative clinical study of the efficacy and safety of the drug, favipiravir, in hospitalised COVID-19 patients conducted in Russia. .
Sample size: 390 patients.
Results
- Median elimination time for the virus was 4 days with favipiravir versus 9 days with standard therapy.
- With favipiravir, day 4 of treatment saw 65% of patients reporting RT-PCR-negative for SARS-CoV-2; day 10 of treatment saw 90% of patients reporting RT-PCR-negative for SARS-CoV-2.
- In the favipiravir group, 68% of the patients reached fever resolution earlier (day 3) versus standard therapy (day 6).
- Overall, reported efficacy of favipiravir was more than 80%.
Safety and Tolerability
Adverse Effects [23]
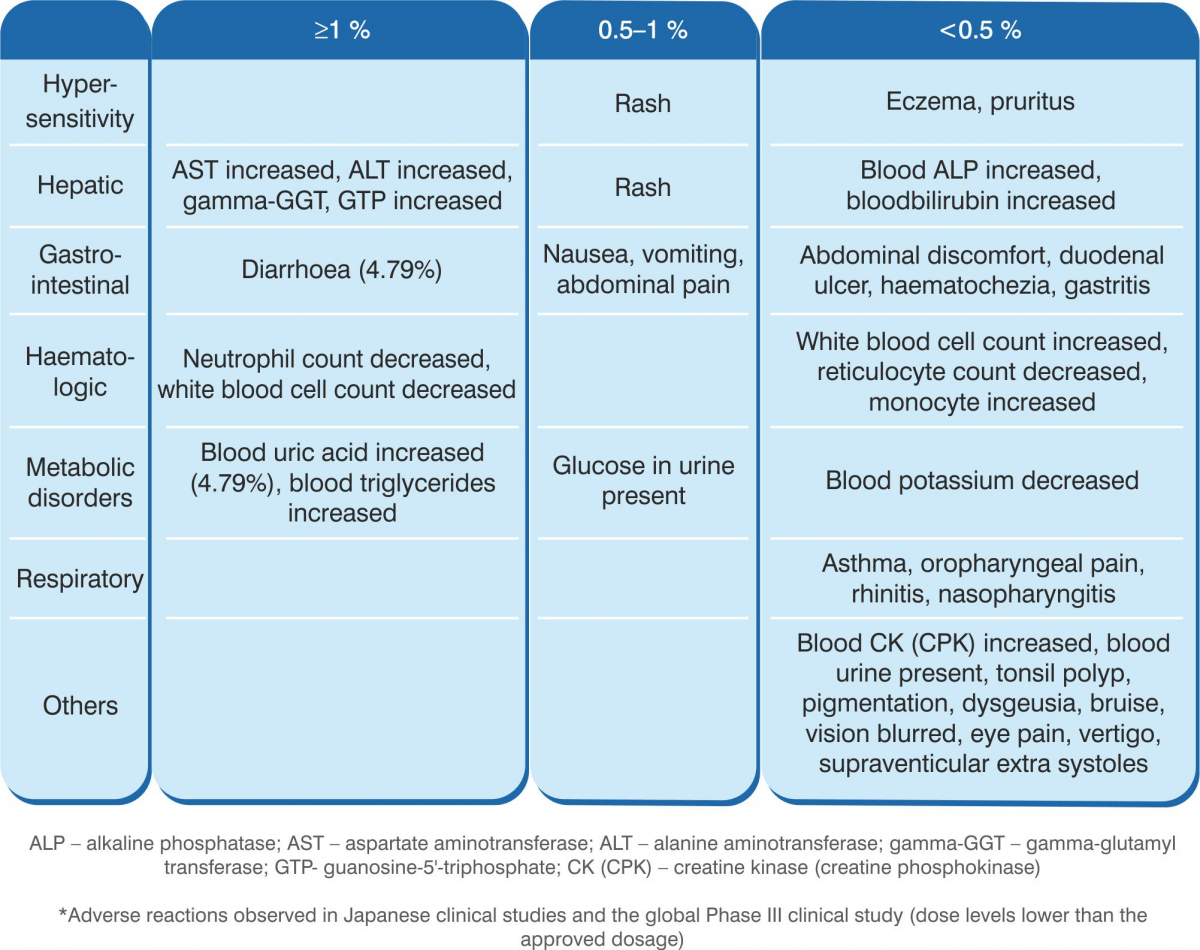
Favipiravir has never been administered with the approved dosage. In Japanese clinical studies and the global Phase III study (studies conducted with dose levels lower than the approved dosage) conducted in influenza, adverse reactions were observed in 100 of 501 subjects (19.96%) evaluated for safety (including abnormal laboratory test values). Major adverse reactions included increase in blood uric acid level in 24 subjects (4.79%), diarrhoea in 24 subjects (4.79%), decrease in neutrophil count in 9 subjects (1.80%), increase in AST in 9 subjects (1.80%), and increase in ALT in 8 subjects (1.60%).
Clinically significant adverse reactions
- The following clinically significant adverse reactions have been reported with other anti- influenza virus agents. Patients should be carefully monitored, and if any abnormality is observed, the treatment should be discontinued and appropriate measures should be taken.
- Shock, anaphylaxis
- Pneumonia
- Hepatitis fulminant, hepatic dysfunction, jaundice
- Toxic epidermal necrolysis (TEN), oculomucocutaneous syndrome such as Stevens-Johnson syndrome (SJS)
- Acute kidney injury
- White blood cell count decreased, neutrophil count decreased, platelet count decreased.
- Neurological and psychiatric symptoms (consciousness disturbed, delirium hallucination, delusion, convulsion, etc.)
- Haemorrhagic colitis
- Abnormal behaviour (frequency unknown): Although a causal relationship is unknown, abnormal behaviour , leading to a fall/accident may occur in patients with influenza virus infection (e.g. suddenly running away, wandering around).
- Other adverse reactions*
If the following adverse reactions occur, appropriate measures should be taken according to the symptoms.
Warnings and Precautions [23]
- Careful administration of favipiravir is necessary in patients with gout or a history of gout, and patients with hyperuricaemia (blood uric acid level may increase, and symptoms may be aggravated.
- Since early embryonic deaths and teratogenicity was observed in pre-clinical studies on favipiravir, do not administer the drug to women known or suspected to be pregnant.
- When administering favipiravir to women of child-bearing potential, it is recommended to confirm a negative pregnancy test result before initiating the treatment course.
- Explain in detail to the woman and her partner about the risks involved in favipiravir treatment, and instruct them to use the most effective contraceptive methods in sexual intercourse during and for 7 days after treatment ends. In case of suspected pregnancy during the treatment, instruct the patient to discontinue the treatment immediately and to consult a physician.
- Favipiravir migrates into semen. When administering the drug to male patients, explain fully the risks involved and given strict instructions about the use of condoms in sexual intercourse during and for 7 days after the end of the treatment. advise them against having sexual intercourse with partners/women who are pregnant or may possibly become pregnant during this period.
- Before starting treatment, explain thoroughly about the efficacy and the risks (including the risk of exposure of the drug to the foetus in pregnancy) to patients or their family members.
- Careful examination of the necessity of favipiravir before use is recommended.
- Pregnant women: Do not administer favipiravir to women known or suspected to be pregnant.
- Breastfeeding: When administering favipiravir to lactating women, instruct them to stop breastfeeding. (The major metabolite of favipiravir, a hydroxylated form, was found to be distributed in breast milk.)
- Paediatric patients: Favipiravir has not been studied in children.
- Geriatric patients: Since the elderly often have reduced physiological functions, favipiravir should be administered with care to them along with regular monitoring their general condition.
Contraindications
Favipiravir is contraindicated in the following patients:
- Women known or suspected to be pregnant (early embryonic death and teratogenecity have been observed in animal studies)
- Lactating women
- Patients with a history of hypersensitivity to favipiravir
- Patients with severe hepatic impairment
- Patients with severe renal impairment
Place in Therapy – Favipiravir
Favipiravir has been approved for Restricted Emergency Use for COVID-19 by the Government of India. Favipiravir tablets are to be used for patients with mild-to-moderate COVID-19. Careful examination of the necessity of favipiravir before use is recommended. The dose is 1,800 mg orally twice daily on day 1, followed by 800 mg twice daily up to a maximum of 14 days. The patient must sign an informed consent before treatment initiation. Since the drug acts by halting viral replication, it is advised to be administered as early as possible after symptom onset. The following benefits are achieved with favipiravir:
- Rapid reduction in viral load
- Faster clinical recovery
- Faster resolution of chest CT changes
- Faster fever and cough resolution
Summary
The ongoing COVID-19 pandemic has affected more than 10 million people worldwide. The cases are surging by the minute and it has a high mortality rate, especially among the elderly and those with comorbid conditions. It has posed a threat not only on the health front but also impacted the social and economic state of countries all over the globe. Government and research centres are working day and night to try and control the spread. Vaccines are in development and a few are in the clinical trial stage. Potential drugs for treating COVID-19 are being evaluated for safety and efficacy.
Currently, antiviral drugs such as remdesivir, favipiravir and other immunomodulators have shown better positive outcomes than others. Hydroxychloroquine and chloroquine were given emergency use authorisation (EUA) by the US Food and Drug Administration (FDA) but it has been revoked on 15 June 2020 due to the risk of heart rhythm problems.
Favipiravir is an antiviral used to treat novel and re-emerging influenza virus infections. It is now being re-purposed for COVID-19 and is undergoing multiple clinical trials in various countries. It has shown positive outcomes in terms of reducing the median time to alleviate symptoms and facilitate early viral clearance in patients with mild-to-moderate disease.
References
COVID-19 Outbreak. World Health Organization; 2020. Available from: https://www.who.int/health-topics/coronavirus#tab=tab_1.
Hubei Timeline. Johns Hopkins University. Available from https://coronavirus.jhu.edu/data/hubei-timeline
Zhang T, Wu Q, Zhang Z. Probable pangolin origin of SARS-CoV-2 associated with the COVID-19 outbreak. Current Biology. 2020 Mar 19.
Coronavirus disease (COVID-2019) situation reports [Internet]. 2020 [cited 2020 May 14]. Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200622-covid-19-sitrep-154.pdf?sfvrsn=d0249d8d_2
Devaux CA, Rolain JM, Raoult D. ACE2 receptor polymorphism: Susceptibility to SARS-CoV-2, hypertension, multi-organ failure, and COVID-19 disease outcome. Journal of Microbiology, Immunology and Infection. 2020 May 6. Available from https://www.sciencedirect.com/science/ article/pii/S1684118220301092
Clerkin KJ, Fried JA, Raikhelkar J, Sayer G, Griffin JM, Masoumi A, Jain SS, Burkhoff D, Kumaraiah D, Rabbani L, Schwartz A. COVID-19 and cardiovascular disease. Circulation. 2020 May 19;141(20):1648-55. https://www.ahajournals.org/doi/pdf/10.1161/CIRCULATIONAHA.120.046941
Mason RJ. Pathogenesis of COVID-19 from a cell biology perspective. Available from: https://erj.ersjournals.com/content/55/4/2000607
Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. Journal of autoimmunity. 2020 Feb 26:102433. Available from https://www.sciencedirect.com/science/article/pii/S0896841120300469
Li X, Geng M, Peng Y, Meng L, Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. Journal of Pharmaceutical Analysis. 2020 Mar 5. Available from https://www.sciencedirect.com/science/article/pii/S2095177920302045
Coperchini F, Chiovato L, Croce L, Magri F, Rotondi M. The cytokine storm in COVID-19: an overview of the involvement of the chemokine/chemokine-receptor system. Cytokine & Growth Factor Reviews. 2020 May 11. Available from: https://www.sciencedirect.com/ science/article/pii/S1359610120300927
Shereen MA, Khan S, Kazmi A, Bashir N, Siddique R. COVID-19 infection: Origin, transmission, and characteristics of human coronaviruses. Journal of Advanced Research. 2020 Mar 16.
Fu Y, Cheng Y, Wu Y. Understanding SARS-CoV-2-mediated inflammatory responses: from mechanisms to potential therapeutic tools. Virologica Sinica. 2020 Mar 3:1-6. Available from https://www.nature.com/articles/s41577-020-0320-7
Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: A clinical–therapeutic staging proposal. The Journal of Heart and Lung Transplantation. 2020 May;39(5):405.
Clinical management of Covid-19. World Health Organization. Available from https://www.who.int/publications/i/item/clinical-management-of-covid-19
Coronavirus disease 2019 COVID-19: Epidemiology, virology, clinical features, diagnosis, and prevention – UpToDate. Apr 2020
Udugama B, Kadhiresan P, Kozlowski HN, Malekjahani A, Osborne M, Li VY, Chen H, Mubareka S, Gubbay JB, Chan WC. Diagnosing COVID-19: the disease and tools for detection. ACS nano. 2020 Mar 30;14(4):3822-35. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7144809/
Clinical Management Protocol. Governement of India MoHFW. Available from: https://www.mohfw.gov.in/pdf/ClinicalManagement ProtocolforCOVID19.pdf
https://cdsco.gov.in/opencms/opencms/en/Latest-Public-Notices/
Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020 May 12;323(18):1824-36. Available from: https://jamanetwork.com/journals/jama/article-abstract/2764727
Furuta Y, Gowen BB, Takahashi K, Shiraki K, Smee DF, Barnard DL. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral Research. 2013 Nov 1;100(2):446-54. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3880838/
Furuta Y, Takahashi K, Kuno-Maekawa M, Sangawa H, Uehara S, Kozaki K, Nomura N, Egawa H, Shiraki K. Mechanism of action of T-705 against influenza virus. Antimicrobial Agents and Chemotherapy. 2005 Mar 1;49(3):981-6. Available from: https://pubmed.ncbi.nlm.nih.gov/15728892/
Shiraki K, Daikoku T. Favipiravir, an anti-influenza drug against life-threatening RNA virus infections. Pharmacology & Therapeutics. 2020 Feb 22:107512. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7102570/
Avigan Tablet 200 mg Prescribing Information Nov 2017 4th version
Cell Research (2020) 0:1–3; https://doi.org/10.1038/s41422-020-0282-0
Cai Q, Yang M, Liu D, Chen J, Shu D, Xia J, Liao X, Gu Y, Cai Q, Yang Y, Shen C. Experimental treatment with favipiravir for COVID-19: an open-label control study. Engineering. 2020 Mar 18. Available from: https://www.sciencedirect.com/science/article/pii/S2095809920300631
Chen C, Huang J, Cheng Z, Wu J, Chen S, Zhang Y, Chen B, Lu M, Luo Y, Zhang J, Yin P. Favipiravir versus arbidol for COVID-19: a randomized clinical trial. MedRxiv. 2020 Jan 1. Available from: https://www.medrxiv.org/content/medrxiv/early/2020/04/15/2020.03.17.20037432.full.pdf
Interim report of the favipiravir (Avigan) observational study. Fujita Health University. Available from: https://www.fujita-hu.ac.jp/en/news/kka9ar0000000ep3.html
https://rdif.ru/Eng_fullNews/5095
Abridged Prescribing Information
CIPLENZA Tablets
Composition: Each tablet contains Favipiravir 200 mg. Indications: For treatment of patients with mild to moderate COVID-19 disease. Dosage & Administration Day 1: 1,800 mg, twice daily. Day 2 onwards: 800 mg, twice daily, up to a maximum of 14 days. The administration should be started promptly after suspected or laboratory confirmation of SARS CoV-2 infection in adults with mild to moderate disease. Contraindications: Pregnant, lactating and women suspected to get pregnant; severe hepatic impairment, severe renal impairment and hypersensitivity to the components of the product. Warnings & Precautions Favipiravir has been proved to be lethal to early embryo as well as to cause teratogenicity in animal studies and, hence, cannot be administered to a pregnant woman or a woman who may pregnant. If the drug is to be administered to a woman with a possibility of becoming pregnant, a pregnancy test should be done before starting the drug to confirm negative pregnancy before starting the drug. In addition, the risks should be fully explained and guidance should be given (to the female patient) accompanied by the partner to ensure implementation of extremely effective contraceptive methods during the administration period and for 7 days after the completion of the administration. Furthermore, if pregnancy is suspected during the administration period of this product, administration should be discontinued immediately and the patient should be instructed to contact a physician. The drug migrates into the semen. Therefore, when administering to male patients, make sure to fully explain the risks and instruct to use extremely effective contraceptive methods during sexual intercourse during the administration period and up to 7 days after the completion of the administration of the drug (men must wear condoms). Also, advise them against having sexual intercourse with partners/women who are pregnant or may possibly become pregnant during this period. Patients with gout or a history of gout and patients with hyperuricaemia (there is a concern of increase in blood uric acid levels and worsening of symptoms). In a clinical study conducted outside Japan to examine the pharmacokinetics of patients with liver dysfunction, plasma concentrations of the drug increased in patients with liver dysfunction. Although the causal relationship is unknown, psychoneurotic symptoms such as abnormal behaviour after administration of anti-influenza virus agents, including favipiravir, have been reported. Bacterial infections can be associated with influenza virus infection or can be confused with flu-like symptoms. In case of a bacterial infection and if a bacterial infection is suspected, take appropriate measures such as administration of antibacterial agents. Drug Interactions Favipiravir is not metabolised by cytochrome P450 (CYP), it is mainly metabolised by aldehyde oxidase (AO), and partly by xanthine oxidase (XO). Furthermore, it inhibits AO and CYP2C8, but has no CYP-inducing effect. Precautions to be followed when administered in combinations with Pyrazinamide, Repaglinide, Theophylline, Famciclovir & Sulindac. Undesirable Effects The main side effects were increase in blood uric acid, diarrhoea, decrease in neutrophil count, increase in AST (GOT) & ALT (GPT). Since the following serious side effects have been reported with other anti-influenza virus drugs, the patient should be carefully monitored and if any abnormalities are found, discontinue administration and take appropriate measures: abnormal behaviour, shock, anaphylaxis, pneumonia, fulminant hepatitis, liver dysfunction, jaundice, toxic epidermal necrolysis (TEN) syndrome, Stevens-Johnson syndrome, acute kidney injury, leucopaenia, neutropaenia, thrombocytopaenia, psychiatric symptoms (disturbance of consciousness, delirium, hallucinations, delusions, convulsions, etc.).
Visit www.ciplamed.com