EASLiver Updates
European Association for the Study of the Liver Berlin . Germany - March 30 - April 3, 2011
Acute-on-chronic liver failure is a serious condition with varied etiology and manifestations, as well as high mortality. Antiviral therapy is recommended in patients with ACLF due to hepatitis B; though the strength of recommendation and quality of evidence for same is not substantial. This retrospective study was designed to evaluate the efficacy and safety of entecavir in 248 patients with HBV-ACLF and to develop a novel model (Tongji prognostic predictory model, TPPM) for prognostic prediction of HBV-ACLF patients.
The patients were divided into two groups ; 124 patients received standard internal medications plus treatment with entecavir; the remaining 124 patients received standard internal medications without nucleoside analogues (NA).
Patient survival; biochemical markers; coagulation capacity; blood routine examination; serology markers; virological markers of HBV-DNA and complications were monitored and included in the multivariate logistical regression analysis upon which the regression model for prognostic prediction (Tongji prognostic predictory model, TPPM) was established.
The 1 - and 3-month survival rates of patients in the entecavir-treated group were significantly higher than that in NA-free group.

The entecavir-treated group also achieved a better improvement of MELD score when compared with the controls.
On multivariate logistic regression, independent predictors of liver-related mortality were high INR for prothrombin time, ≥2 complications and high total bilirubin, but not HBV DNA.
The TPPM scoring offered a better prediction value in both specificity and sensitivity for 3-month mortality of patients with HBV-ACLF compared with MELD scoring system with statistically significant difference.
In the patients with HBV-ACLF, using a cutoff of 0.22 for 3-month predicted mortality by TPPM, the positive predictive value was 93.6%, with a negative predictive value of 91.3%.
The established TPPM scoring system offers superior predictory value in both specificity and sensitivity for HBV-ACLF patients when compared with MELD
Entecavir treatment prevents disease progression and increased the survival of patients with HBV-ACLF
K. Ma, W. Guo, Q. Ning et al. Poster Presentation no. 742 presented at EASL 2011.
Prospective cohort study assessed the efficacy, drug-resistance, and development of hepatocellular carcinoma (HCC), in a long-term nucleos(t)ide analogue (NA) treatment with lamivudine (LVD) and entecavir (ETV), for chronic hepatitis B (CHB) and cirrhosis.

Incidence of drug-resistance was 60 cases in LVD group, and only 1 in ETV group. When LVD-resistance developed, co-administration of adefovir-dipivoxil (ADV) or change to ETV was selected. The subjects were prospectively followed-up with monthly blood test for virology and biochemistry, including alpha-fetoprotein (AFP) or des-y-carboxy prothrombin (DCP). Ultrasonography (US), enhanced CT, or MRI, was performed 6-monthly in CHB and 3-monthly in cirrhosis.
After NA treatment, median serum HBV-DNA reduced significantly from 7.0 to 2.1 log copies/mL, ALT (from 87 to 19 IU/L), and AFP (from 5.8 to 2.9 ng/mL) (p< 0.0001), while albumin (from 4.0 to 4.4 g/dL) and prothrombin time (from 88 to 100%) were significantly elevated (p< 0.0001), with improvement in Child-Turcotte-Pugh class (p=0.0173).
HCC developed in 35 patients with stage 1/2/3/4A in 15/14/5/1 patients. The incidence of HCC at years 1/3/5/7/10 was significantly (p< 0.0001) higher in cirrhosis (7.94/ 17.2/ 42.1/ 45.6/ 52.4 %) than CHB (1.56/ 3.46/ 3.46/ 7.1/ 28.5 %), with no difference between ETV and LVD.
After NA treatment, sensitivity of AFP became higher than that of baseline (64.4%). After NA treatment, serum AFP dropped to lower than 10 ng/mL with marked elevation of specificity, leading to an earlier detection of HCC.
Long-term nucleos(t)ide analogue treatment showed a sustained efficacy and improved hepatic reservation in CHB and cirrhosis
H. Kobashi et al. Poster Presentation no. 727 presented at EASL 2011.
A major concern with nucleoside analogs (NA's) treatment is the selection of antiviral-resistant mutations. Long term therapy with NA's, in particular, is associated with an increasing risk of the development of multi drug resistance, making long-term viral suppression vital to prevent hepatic flares in these patients.
This ongoing open label multicenter cohort study investigates the efficacy and safety of entecavir (1 mg) and tenofovir combination used as rescue therapy in 55 adherent HBV-infected patients with multidrug resistant HBV or only partial responses to previous lines of therapy and advanced liver disease.
Quantitative HBV-DNA measurement with LLOD< 69 lU/ml was used. Resistance was determined using Innolipa line-probe-assay DRV3 and direct sequencing. ALT and HBV-DNA were measured at baseline and every 3 months.
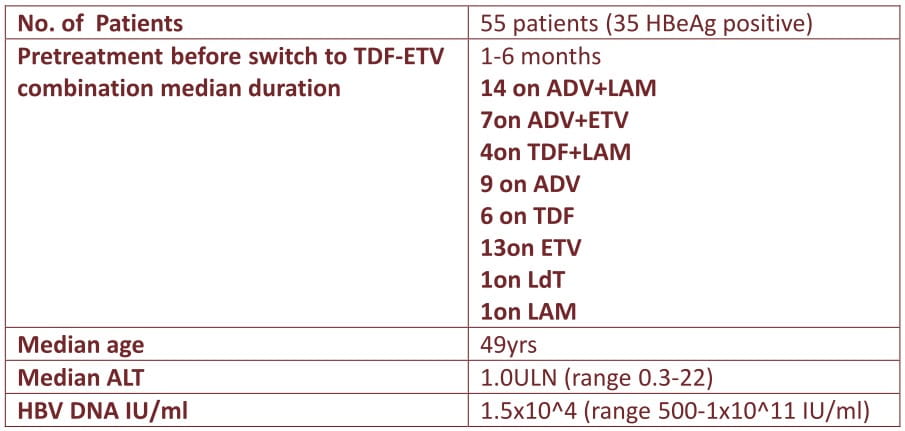
Median treatment duration of TDF-ETV combination therapy was 18 months (range 3-57 months, October 2010) during which median HBV-DNA level dropped by 3 logs (range 0-8 log; p< 0.0001).
49/55 patients became HBV-DNA undetectable, median after 6 months (95%CI 4.6-7 month).
Probability of reaching complete HBV DNA suppression was not decreased in patients with ADV or ETV resistance.
Ongoing viral suppression was accompanied by decline in ALT (median 0.7 ULN; range 0.2-2.4; p=0.001).
Four patients lost HBeAg (after 15, 18, 21, and 24 months, respectively), one patient showed HBs-seroconversion.
Besides long-term viral suppression (21 and 37 month) two patients showed significant viral rebound.
Patients with liver cirrhosis did not develop clinical decompensation, but two patients with cirrhosis and undetectable HBV DNA developed HCC's. There were no significant clinical or renal side effects and no lactic acidosis.
HBV infected patients with advanced liver disease harbouring complex viral resistance patterns or showing partial antiviral responses to preceeding therapies show good response to rescue therapy with entecavir and tenofovir
J. Petersen et al. Poster Presentation no. 744 presented at EASL 2011.
Entecavir is currently the recommended firstline drug in for treatment-naive chronic hepatitis B (CHB) patients ; long-term data of uninterrupted entecavir up to 4 years however is lacking.
In this study, 222 CHB patients were treated continuously with entecavir 0.5mg daily for up to 4 years. The cumulative rates of HBeAg seroconversion, ALT normalization, DNA undetectability and entecavir signature mutations up to year 4 were determined. HBV DNA levels were measured by Roche Taqman real time PCR assay (lower limit of detection: 12 lU/mL). Resistance profile was determined by line probe assay (LiPA) for patients with detectable HBV DNA.
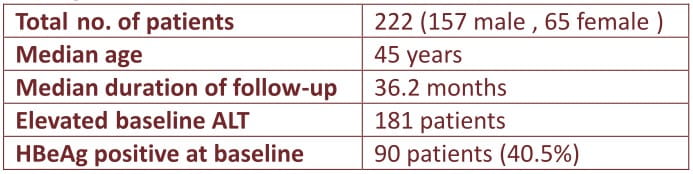
Excellent virologic, biochemical and HBeAg seroconversion seen during 4 years treatment
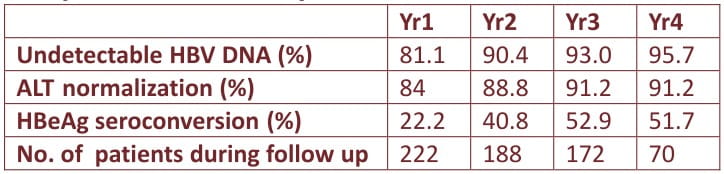
One patient developed HBsAg seroconversion at year 2
Virologic breakthrough (>1 log HBV DNA increase from the nadir) was noticed in 4 patients; 1 patient developed entecavir signature mutation at year 3, resulting in a cumulative resistance of 0.6% up to year 4. There were no serious adverse events related to the drug
Continuous entecavir up to 4 years achieved a more than 95% chance of undetectable HBV DNA and only a 0.6% probability of resistance
W. -K. Seto et al. Poster Presentation no. 748 presented at EASL 2011.
Entecavir (ETV) is a potent inhibitor of viral replication in chronic hepatitis B patients. To date, there is no published data concerning the use of ETV in nucleoside (NUC) naive HBsAg-positive renal transplant recipients (RTRs).
We prospectively treated 21 RTRs positive for HBsAg with 0.5 mg of ETV daily since 2007, with a mean duration of 95.3 ± 33.9 weeks (range, 46-161). Serial HBV DNA is assessed at baseline, week 12, 24, 52, and week 104 after ETV treatment. Another cohort of 19 patients who have received complete 2-yr lamivudine (LAM) therapy during 2004-2007 was used as a historical control for comparison.
Of the 21 RTRs, 12 (57%) were treatment naive patients and the other 9 (43%) were LAM experienced without YMDD mutations.
By comparison with LAM treated cohort, the percentage of undetectable HBV DNA in ETV-treated RTRs was significantly higher than LAM-treated RTRs at every time point (P < 0.005) without evidence of viral resistance.
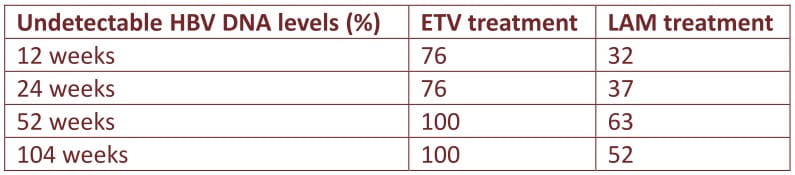
By excluding the 9 patients who were LAM experienced, 12 ETV-naive RTRs still exhibited a better virologic response at 52 and 104 weeks than LAM-na'i've RTRs (P= 0.035).
In the 9 patients who overlapped in two cohorts, ETV therapy exhibited a more rapid virologic response than their previous LAM therapy at every time point, especially at 12 and 24 weeks (P = 0.009).
There was no significant change of GFR and no patients developed lactic acidosis or myopathy during treatment.
Entecavir is efficient for treating chronic hepatitis B after renal transplantation, and is superior to lamivudine in view of drug related renal graft dysfunction, lactic acidosis, myopathy, as well as virologic resistance
M.-C. Tsai, et al. Poster Presentation no. 752 presented at EASL 2011.
Entecavir (ETV) is a potent inhibitor of hepatitis B virus (HBV) replication. The aim of this study was to investigate the long-term efficacy and safety of 333 HBV monoinfected patients (mean age 43 ±14 years; 248 (75%) male; 143 (44%) HBeAg+; mean HBV DNA 6.2±1.7 log lU/ml) treated with ETV monotherapy, particularly in those with previous adefovir treatment from ten large European referral centers.
Serum HBV DNA and ALT were measured every 3 months. Virological response (VR) was defined as serum HBV DNA < 80 lU/mL and virological breakthrough (VB) as a confirmed >1 log increase in HBV DNA from the nadir.
243 patients were nucleos(t)ide (NA)-naive, while 90 had been previously exposed to NA. Median follow up of the study population was 20 (3-51) months.
191 of 237 (81%)treatment naive patients achieved virologic response(VR).
43 of 51 ADV-experienced (+LAM in 29 (67%)) patients who were directly switched to ETV.
Twelve (28%) patients had a history of genotypic resistance to ADV (rtN236T/ rtA181V/T) with a median ADV treatment duration of 18 (3-55) months.
22 (88%) ADV-experienced patients without LAM-resistance achieved VR during 32 (12-46) months of follow up. One patient experienced virological breakthrough and developed genotypic ETV resistance.
72 patients were LAM-experienced among which 51% patients had a history of LAM-resistance. Forty-two (58%) of these patients achieved VR during 18 (3-51) months of follow up.
Adjusted for previous LAM treatment and resistance, baseline viral load and HBeAg status, antiviral response to ETV was neither influenced by prior ADV therapy (aHR 0.84; 95% CI 0.50-1.41; p=0.51) nor by previous ADV-resistance (aHR 1.33; 95% CI 0.59-2.97; p=0.49) (see figure).
No major side effects, such as nephrotoxicity, were seen during ETV therapy.
[aHR for achieving virological response]
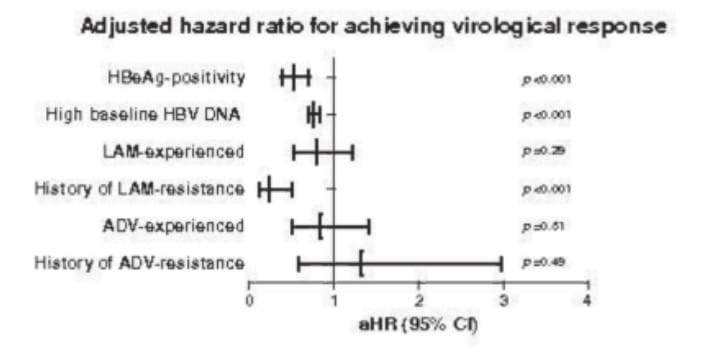
Entecavir monotherapy is safe and effective in adefovir-experienced patients in the absence of lamivudine resistance
R. Zoutendijk et al. Poster Presentation no. 760 presented at EASL 2011.
Life-long antiviral therapy with nucleos(t)ide analogues is required in chronic hepatitis B (CHB) patients with liver damage. Data on long-term efficacy, drug resistance and safety of de-novo combination therapy with lamivudine (LAM) 100mg/d + adefovir (ADV) 10mg/d in naive CHB as compared to potent antivirals like entecavir used as monotherapy is limited. The potential benefits of using combination therapy include synergistic added antiviral benefit with reduced antiviral resistance however long term safety, adherence issues and potential drug interactions can prove to be detrimental.
To assess the long-term impact of de-novo LAM+ADV versus entecavir (ETV) monotherapy (0.5mg/d) on virological response and renal/bone safety in treatment naive 346 CHB patients treated for a median of median 30 months.
Patients were divided into 2 groups: LAM+ADV (n=192, 78% males, median age 40y, 35% HBeAg+, 34% cirrhosis) and ETV (n=154, 79% males, median age 42y, 31% HBeAg+, 34% cirrhosis).
HBeAg seroconversion and complete virological response (CR) (HBV DNA< 12 lU/ml) was compared between groups. Renal/ bone safety was assessed with creatinine and phosphate levels and estimated glomerular filtration (eGF) at each time-point and compared between groups and with baseline. HBV viral load in all patients and HBV genotypic resistance was tested in all patients with suboptimal response by direct sequencing.
Baseline HBV DNA was similar in LAM+ADV and ETV groups (median log10 4.6 vs. 4.5 IU/ml) and similar proportions achieved complete response(CR) but HBeAg seroconversion was more frequent in LAM+ADV than ETV (21%vs.6%/p=0.02).
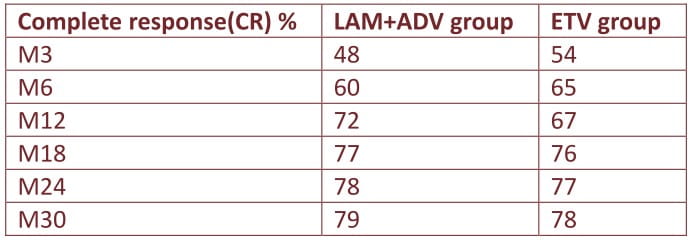
HBV DNA decrease was more profound in LAM+ADV than ETV from M24 (log10 5.03vs.4.62IU/ml, p=0.05).
No viral mutations associated with drug resistance were detected in LAM+ADV than in ETV group (rtM204l mutation).
No significant differences in serum creatinine or eGF between groups at each time-point, however there was significant decrease in eGF from M18 onwards in LAM+ADV group (82.2vs.76.2ml/s,p=0.04) vs. baseline values.
At month 12, serum phosphate levels fell significantly on therapy in the LAM+ADV but not the ETV group as shown in table below
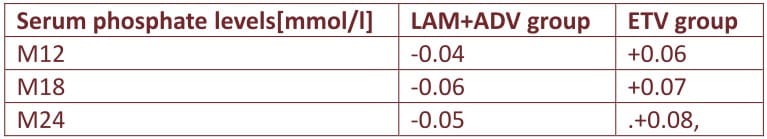
De-novo combination LAM+ADV therapy has advantages of similar rate of complete response, higher degree of HBeAg seroconversion; stronger HBV DNA suppression from M24, no drug resistance mutations as compared to entecavir however higher decrease in eGF from M18 compared to baseline and greater drop in phosphate levels from M12 than ETV monotherapy thus a negative impact on renal/bone safety
I. Carey, A. Mendes, D. Joshi, M. Et al. Poster Presentation no. 707 presented at EASL 2011.