3-day Treatment with Azithromycin (10 mg/kg per day once daily), A Promising Alternative Therapy for Tonsillitis in Children
Introduction
Tonsillitis is the most common upper respiratory infection disease in children, mainly caused by Group A ?-hemolytic streptococcus (GAS)infection. Azithromycin is a type of macrolide antibiotic and used for the treatment of varieties of bacterial infections, including pharyngitis and tonsillitis.
Aim
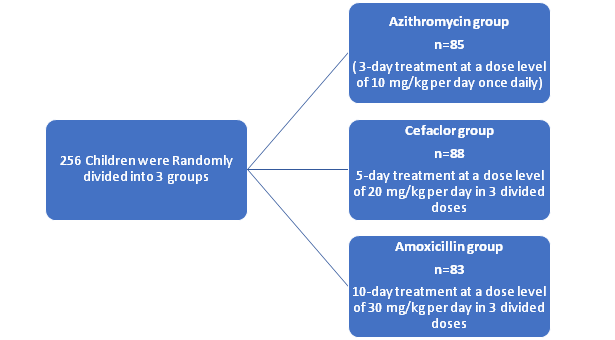
To evaluate and compare the clinical efficacy of azithromycin (10 mg/kg per day once daily for 3 days) or cefaclor (20 mg/kg per day in 3 divided doses for 5 days)
with amoxicillin (30 mg/kg per day in 3 divided doses for 10 days), for the treatment of tonsillitis in children.
Patient Population
- N=256 children
- aged between 2 and 12 years
- signs and symptoms of tonsillitis
- The children were diagnosed with tonsillitis by the presence of a sore throat and (or) a swollen, hyperemic, or suppurated tonsil and (or) visible secretions or erythema of tonsil and (or) with fever
Methods
- Each patient was scheduled for the follow-up for the clinical and bacteriologic assessment on day 14 and day 30 from the start of the treatment
- The signs and symptoms were observed, and the tonsils swab cultures were conducted for the bacteriological examination
- Blood sample and urinalysis were performed to determine the safety and the complication of the drug for the patients
- Clinical and Bacteriological assessments were conducted
- The therapeutic efficacy was evaluated as success or failure on day 14 from the start of the treatment according to the clinical symptoms
- The clinical success was defined by the improvement of signs and symptoms and the disappearance of fever, such that no additional drugs were needed for the therapy
- The therapy failure was defined by the lack of improvement in clinical symptoms, which needed additional antibiotic therapy
- The first bacteriologic outcome was defined as eradication or failure by a pharyngeal/tonsil swab culture on day 14, soon after completion of therapy
- The success of microbiologic evaluation was defined by the eradication of the pathogen identified by a pharyngeal/tonsil swab culture
- The secondary microbiologic outcome was evaluated on day 30 among patients designated microbiologic successes on day 14 and with the follow-up at day 30, which was classified as eradication and recurrence (the reappearance of the pathogen
Results
|
|
p | |||||
|
Azithromycin vs. |
Azithromycin vs. |
Cefaclor vs. | ||||
|
Variable |
Azithromycin |
Cefaclor |
Amoxicillin |
cefaclor |
amoxicillin |
amoxicillin |
|
No. of patients |
85 |
88 |
83 |
|
|
|
|
Age (years) |
5.6±2.3 |
5.8±2.3 |
5.9±2.4 |
0.568a |
0.409a |
0.781a |
|
Sex (male/female) |
40/45 |
49/39 |
45/38 |
0.257b |
0.354b |
0.847b |
|
Mass (kg) |
20.6±7.1 |
22.1±7.8 |
20.9±6.4 |
0.188a |
0.774a |
0.273a |
|
Duration of infection before treatment (days) |
2.1±0.7 |
2.2±0.9 |
2.3±1.0 |
0.416a |
0.135a |
0.493a |
aStudent’s t-test.
b x2 test.
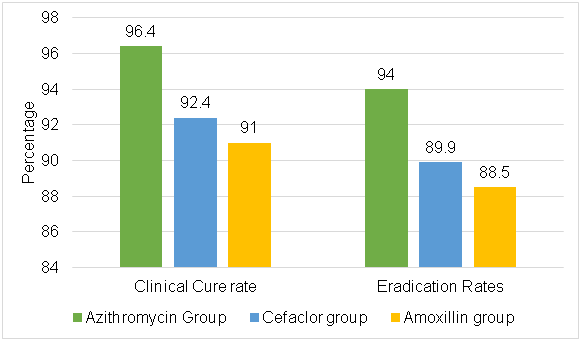
Clinical Efficacy and Microbiologic Outcome
- At the end of therapy (day 14) and microbiologic results at follow-up (day 30)
- There was no statistical difference, but a tendency toward more chance of therapeutic improvement in patients treated with azithromycin in comparison with those treated with cefaclor and amoxicillin was observed
|
Clinical efficacy and microbiologic outcome |
No. of patients (%) |
P | ||||
|
Azithromycin vs. |
Azithromycin vs. |
Cefaclor vs. | ||||
|
Azithromycin |
Cefaclor |
Amoxicillin |
Cefaclor |
amoxicillin |
amoxicillin | |
|
Clinical Efficacy |
n=83 |
n=79 |
n=78 |
|
|
|
|
Success |
80 (96.4) |
73 (92.4) |
71 (91.0) |
0.3198a |
0.2002a |
0.7538b |
|
Failure |
3 (3.6) |
6 (7.6) |
7 (9.0) |
|
|
|
|
Microbiologic outcome at 14 day |
n=83 |
n=79 |
n=78 |
|
|
|
|
Eradication |
78 (94.0) |
71 (89.9) |
69 (88.5) |
0.3367b |
0.2146b |
0.7759b |
|
Failure |
5 (6.0) |
8 (10.1) |
9 (11.5) |
|
|
|
|
Microbiologic results at day 30 |
n=77 |
n=71 |
n=68 |
|
|
|
|
Eradication |
75 (97.4) |
66 (93.0) |
64 (94.1) |
0.261a |
0.419a |
0.947b |
|
Failure |
2 (2.6) |
5 (7.0) |
4 (5.9) |
|
|
|
aFisher’s exact test.
bx2 test.
Safety
- The adverse events occurrence rate in the azithromycin treatment group was lower than those in the other 2 groups (p = 0.030 and p = 0.029)
- No significant difference was observed between cefaclor and amoxicillin groups (p = 0.977)
- Azithromycin treatment showed lower occurrence rate of adverse events than the other 2 groups
|
|
No. of patients (%) |
P |
|||||
|
Azithromycin vs. cefaclor |
Azithromycin vs. amoxicillin |
Cefaclor vs. amoxicillin |
|||||
|
Variable |
Azithromycin |
Cefaclor |
Amoxicillin |
||||
|
Adverse events |
n=83 |
n=80 |
n=79 |
|
|
|
|
|
Diarrhea |
1 (1.2) |
2 (2.5) |
4 (5.1) |
0.616a |
0.202a |
0.443a |
|
|
Rash |
0 (0) |
5 (6.3) |
2 (2.5) |
0.027a |
0.236a |
0.443a |
|
|
Nausea |
1 (1.2) |
2 (2.5) |
3 (3.8) |
0.616a |
0.358a |
0.358a |
|
|
Total events |
2 (2.4) |
9 (11.3) |
9 (11.4) |
0.030a |
0.029a |
0.977b |
|
aFisher’s exact test
b X2 test
Conclusion
- The study demonstrated that azithromycin showed a better tendency of clinical efficacy and microbiologic outcome with lower recurrence of bacteria and less adverse events compared with the other 2 groups
- The three groups showed no statistical difference for the clinical effectiveness and bacterial elimination, however, there was a tendency that azithromycin treatment is more effective than the cefaclor and amoxicillin treatments with a lower occurrence rate of adverse reactions
- The findings indicated 3-day treatment with azithromycin (10 mg/kg per day once daily) is a promising alternative for therapy of tonsillitis in children caused by GAS
Reference
Can J Physiol Pharmacol.2019;97(10):939-944