Introduction
The angiotensin receptor–neprilysin inhibitor (ARNI) sacubitril–valsartan is associated with reduced risk of hospitalization for heart failure (HHF) or death from cardiovascular (CV) causes in patients with HF and reduced ejection fraction (HFrEF). Nevertheless, little is known about the impact of ARNI in patients with HF with preserved ejection fraction (HFpEF).
Aim
To determine whether sacubitril–valsartan would lower the rate of a composite outcome of total HHF and death from CV causes vs. valsartan in patients with HFpEF.
Patient Profile
-
Patients with New York Heart Association (NYHA) class II to IV HF, with EF ≥ 45% within previous 6 months (n=4822)
-
All patients had elevated levels of natriuretic peptides, and structural heart disease, and were on diuretic therapy.
Methods
Study Design
-
The Prospective Comparison of ARNI with ARB [angiotensin-receptor blockers] Global Outcomes in HFpEF (PARAGON-HF) trial was a randomized, double-blind, active-comparator trial.
Treatment Strategy
-
All patients first received valsartan at half the target dose, followed by sacubitril–valsartan at half the target dose during the single-blind run-in period.
-
Run-in period was followed by a double-blind treatment period during which patients without unacceptable side effects in both run-in phases and whose laboratory values remained within prespecified safety criteria were randomized 1:1 to receive one of the following:
-
Sacubitril–valsartan (target dose, 97 mg of sacubitril and 103 mg of valsartan twice daily)
-
Valsartan (target dose, 160 mg twice daily)
Outcomes
Primary Outcomes
-
Composite of total HHF and death from CV causes
Secondary Outcomes
-
Change in NYHA class
-
Worsening renal function
-
Change in Kansas City Cardiomyopathy Questionnaire (KCCQ) clinical summary score (scale, 0 to 100, with higher scores indicating fewer symptoms and physical limitations),
Safety Outcomes
-
Incidence of hypotension, renal dysfunction, hyperkalemia, and angioedema
Follow-up
-
Median follow-up period of 35 months
Results
-
The final efficacy analysis included 4796 patients.
-
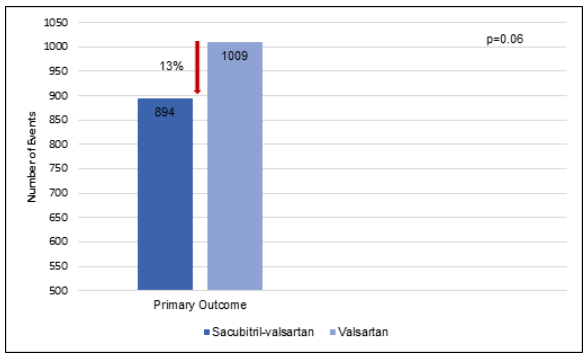
Treatment with sacubitril-valsartan vs. valsartan reduced the incidence of primary outcomes significantly by 13% (rate ratio, 0.87; P=0.06) (Fig. 1).
Fig. 1: Primary Outcomes during the study
-
The incidence of death from CV causes reduced insignificantly by 5% in the sacubitril–valsartan vs. the valsartan group [8.5% vs. 8.9%; hazard ratio (HR): 0.95; 95% CI: 0.79 to 1.16]
-
The incidence of total HHF reduced by 15% in the sacubitril-valsartan vs. valsartan group (690 vs. 797; rate ratio: 0.85; 95% CI: 0.72 to 1.00).
-
A greater proportion of patients treated with sacubitril-valsartan had improvement in the NYHA class [15.0% vs. 12.6%; odds ratio (OR), 1.45; 95% CI, 1.13 to 1.86].
-
Treatment with sacubitril-valsartan vs. valsartan reduced the worsening of renal function by 50% (1.4% vs. 2.7%; HR: 0.50; 95% CI, 0.33 to 0.77).
-
Patients in the sacubitril–valsartan vs. valsartan group had a 1.0 point higher mean change in the KCCQ clinical summary score at 8 months (95% CI, 0.0 to 2.1).
-
A higher proportion of patients in the sacubitril–valsartan group vs. the valsartan group had an improvement of ≥5 points in the KCCQ clinical summary score (33.0% vs. 29.6%; OR: 1.30; 95% CI: 1.04 to 1.61).
-
The incidence of hypotension and angioedema was higher and that of hyperkalemia was lower in patients treated with sacubitril–valsartan.
-
Analysis of 12 prespecified subgroups, suggested of possible benefit with sacubitril–valsartan in patients with lower EF and in women.
Conclusions
-
Treatment with sacubitril–valsartan resulted in fewer primary outcomes events, compared with valsartan in patients with HFpEF.
-
There was a modest but statistically nonsignificant, lower rate of HHF with sacubitril–valsartan, compared with valsartan and no significant difference in the risk of death from CV causes.
-
The change in the NYHA class from baseline to 8 months and the incidence of worsening renal function were in favor of sacubitril–valsartan over valsartan.
N Engl J Med 2019;381:1609-20.