A Comparison of Lung Deposition and Device Resistance of Two Discrete Multidose Dry Powder Inhalers: Multihaler? (60 doses) and Accuhaler?
26 Apr, 18
Background
- The MuIti-haler TM is an advanced discrete multidose-DPI (mDPI) developed to match the performance of an established mDPI, Accuhaler. It contains 60 doses, has an in-built dose counter with a new design.
- Development of new multi-dose dry powder inhalers (mDPIs) comparable to existing ones is an important challenge. One of the key challenges in producing a bioequivalent test DPI drug product is to manufacture a powder formulation with functionality to enable comparable in vitro performance of the test device to the reference device.
- The objective of this study was to compare the performance of mDPIs: Accuhaler (reference product) and Multi-haler TM (test product) by measuring the device resistance and in-vitro lung deposition at different flow rates of 30, 60 and 90 L/min.
- The in-vitro performance parameter used was percent fine particle mass (%FPM). The %FPM is the FPM expressed as the percentage of the delivered dose. Thus, %FPM gives an indirect estimation of the drug deposition in the lungs.
- The present study compared the performance of the two devices by measuring the device resistance and in-vitro lung deposition at different flow rates of 30, 60 and 90 L/min.
Methods
- The Test-mDPI (Seroflo 250 Multi-haler TM, Cipla Ltd) and Reference-mDPI (Seretide 250 Accuhaler, GlaxoSmithKline Pharmaceuticals Ltd.) were evaluated for performance parameters of device resistance and an in-vitro lung deposition in form of percent FPM at three different flow rates of 30, 60 and 90 L/min.
- Three random samples of Test-mDPI and Reference-mDPI were evaluated for the above performance parameters.
- The %FPM was measured by Next Generation Impactor (NGI, Copley Scientific).
- Percent fine-particle mass (%FPM) is the percentage of the mass found on the stages of the impactor having a cut off diameter of less than 5 microns relative to the label claim.
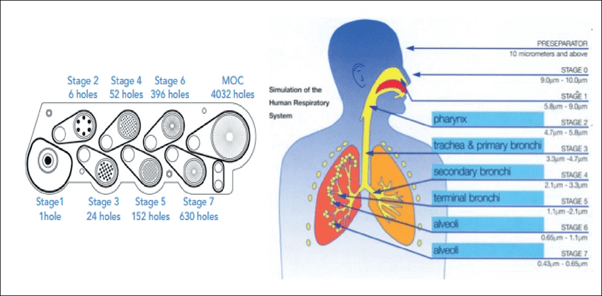
- NGI mimics the human respiratory tree and has a horizontal planar layout adopted for ease of operation and automation. It has seven stages, five of which are in the range 0.5 to 5 microns plus a micro-orifice collector which acts as a final filter (Figure 1). The air flow passes through the impactor in a saw tooth pattern.
Figure 1: The NGI Cascade and simulation of human respiratory system
Procedure
- In vitro characterization of aerosolization performance of test and reference DPI products for the study was performed using a Next Generation Impactor (NGI) with a pre-separator, which was connected to a vacuum pump.
- For each experiment, three individual shots were discharged into the NGI using the reference or test device at 30, 60 and 90 L/min for duration of time representative of an inhaled volume of 4 L.
- The mass of drug deposited on each stage, mouthpiece, induction port and pre-separator, including the device, was determined by high performance liquid chromatography (H PLC).
- Device resistance was measured using Critical Flow Controller (TPK 2000, Copley Scientific).
Results
- In-vitro Lung Deposition
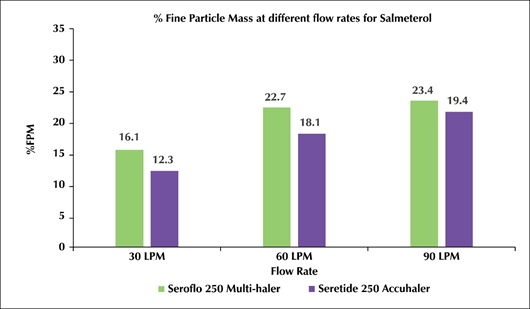
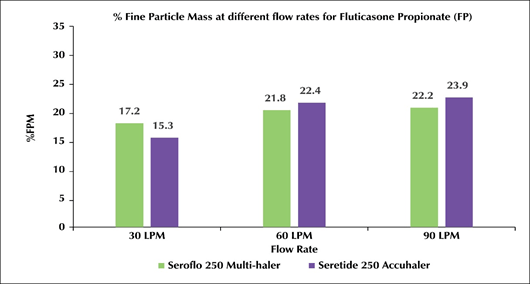
- The %FPM for Test-mDPI at 30, 60 and 90L/min for salmeterol was 16.1, 22.7 and 23.4 while fluticasone propionate was 17.2, 21.8 and 22.2 respectively.
- The %FPM for Reference-mDPI at 30, 60 and 90L/min for salmeterol was 12.3, 18.1 and 19.4 while fluticasone propionate was 15.3, 22.4 and 23.9 respectively (Figure 2).
Figure 2: Test-mDPI (Seroflo 250 Multi-Haler) Vs reference-mDPI (seretide 250 accuhaler) at various flow rates
- Device Resistance
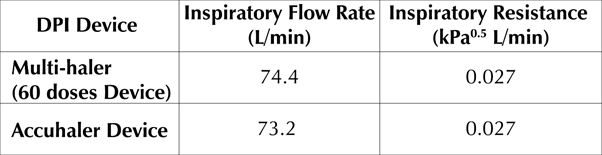
- The inspiratory resistance for Test-mDPI and Reference-mDPI were found to be similar at 0.027(kPa°5 L/min) and 0.027(kPa°5 L/min) which correspond to an inspiratory flow rate of 74.4L/min and 73.2L/min respectively
Table 1: Resistance and inspiratory flow rate - Multi-halerTm (60 Doses) Vs Accuhaler
Discussion
- Percent FPM gives an indirect estimation of drug delivery to the lungs.
- The %FPM values at 60 and 90 L/min were almost similar for both the active ingredients. At 30L/min the % FPM is slightly lower at 30 L/min.
- The %FPM values for both Test-mDPI and Reference-mDPI at 30, 60 and 90 L/min were not significantly different for both the active ingredients.
- At 30L/min, the %FPM was slightly lower for both devices when compared with 60 and 90 L/min flow rates.
- Devices Resistance is a critical attribute in controlling the airflow that de-agglomerates and transports the dry powder aerosol into the lungs.
- When a patient inhales through a DPI, turbulent energy inside the device is created by the pressure drop that results from the interaction between the patient's inhalation flow and the internal design of the DPI, which translates into a resistance to airflow.
- The higher the resistance of the DPI, the lower the inspiratory flow required to generate an adequate dose and vice versa.
- The resistance of both, the test and reference DPI was found to be same i.e. medium resistance devices.
Conclusion
- The present study shows similar in-vitro comparability achieved for two discrete multidose devices, Test-mDPI and Reference-mDPI.
- Hence, the Test-mDPI and the Reference-mDPI have similar performance characteristics in-vitro and meets the performance requirements of the Indian Pharmacopoeia.
References
- Pharmacotherapy 2010; 30 (6): 562584
- J Aerosol Med 1993, 6: 99-110
- Respir Med. 2003; 97:181-87
- Int J Pharm 2016; 513, 294-301
- Respi Med 2014; 108, 1195-1203
- Multidisciplinary Respiratory Medicine 2015 10:13
- AAPS J. 2012; 14:667-76.