More than 1/4th diabetic hypertensives not responding to ACE inhibitor/ARB monotherapy attain BP goals with addition of amlodipine.
ADHT (Amlodipine Diabetic Hypertension Efficacy Response Evaluation Trial)
18 Mar, 14
ADHT
Background
Aim
To determine the efficacy & safety of amlodipine in diabetic hypertensives who do not respond to ACE inhibitor or ARB monotherapy.
Study Patients
Patients with hypertension & diabetes who did not reach target BP goals with ACE inhibitor/ARB monotherapy (N=411).
Study Groups
- ACE inhibitor (quinapril 20-40 mg/day) + Amlodipine (5-10 mg/day) [n=96]
- ACE inhibitor (quinapril 20-40 mg/day) + Placebo [n=103]
- ARB (losartan 50-100 mg/day) + Amlodipine (5-10 mg/day) [n=115]
- ARB (losartan 50-100 mg/day) + Placebo [n=97]
Study Period
12 weeks
Primary Efficacy Outcome
Percentage of patients with BP <130/80 mmHg
Results
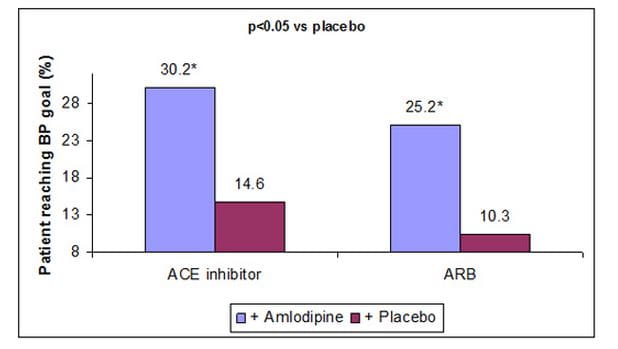
- Addition of amlodipine to ACE inhibitor/ARB monotherapy more than doubled the percentage of patients attaining BP goals vs placebo (Figure 1)
Figure 1: Percentage of patients achieving BP goal (<130/80 mmHg) with add-on amlodipine therapy vs placebo
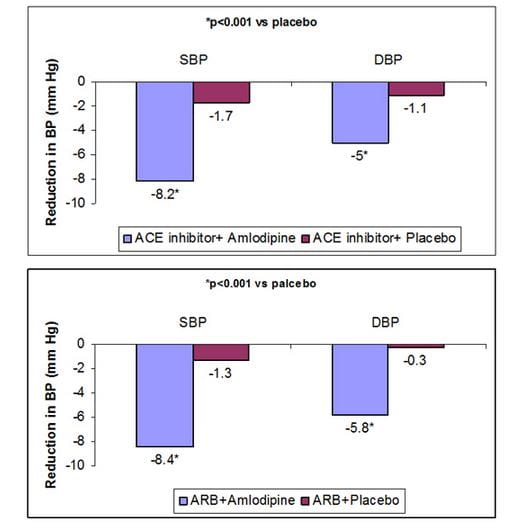
- Addition of amlodipine also yielded significantly greater reductions in systolic & diastolic BP vs placebo (Figure 2)
Figure 2: Reduction in BP with add-on amlodipine therapy vs placebo
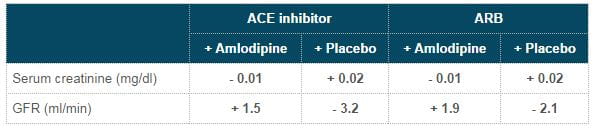
- Serum creatinine levels & glomerular filtration rate (GFR) improved with addition of amlodipine unlike placebo (Table 1)
Table 1: Effect on serum creatinine & GFR
Safety
There was no significant difference in adverse events across the study groups.
Conclusion
Addition of amlodipine in diabetic hypertensives not responding to ACE inhibitor/ARB monotherapy may be a good option for intensive BP lowering to achieve target BP goals.
Ann Pharmacother. 2008 Nov;42(11):1552-62