Amoxicillin and Cefpodoxime Demonstrate Similar Clinical Efficacy in Children with Pneumonia
16 Jun, 20
Introduction
The mortality rate of pneumonia in children below 5 years is significant in Bangladesh. Amoxicillin and Co-trimoxazole have been recommended by WHO as first line drugs for the treatment of pneumonia. However, the emergence of resistance to these antimicrobial drugs has led to the need of an alternative antibiotic for the treatment of pneumonia. Cefpodoxime seems an appropriate choice in the empirical treatment of community acquired respiratory tract infections.
Aim
The effect of oral Amoxicillin and Cefpodoxime proxetil has been compared in children with pneumonia.
Methods
Study Design
- Prospective, randomized, open-label study
Treatment Strategy
- A total of 197 children diagnosed with pneumonia were enrolled, out of which 174 completed the trial
- Amoxicillin 30 mg/kg/day thrice daily was given to 85 children with a mean age of 20.16 months for 5 days
- Eighty nine children with a mean age of 15.75 months received cefpodoxime proxetil 10 mg/kg/day twice daily for 5 days
- The cohort was followed up on day 3 and 5 after the initiation of treatment
Endpoints
- Changes in the following parameters
- Duration of cough
- Duration of dyspnea
- Duration of fast breathing
- Mean heart rate
- Mean temperature
- Mean respiratory rate
- Treatment outcome
Results
- Both the therapy groups had similar baseline clinical characteristics
- Significant improvements in mean body temperature, respiratory rate and wheeze were reported both the groups after the treatment (p <0.001).
- The differences in the response to therapy were insignificant (p >0.127)
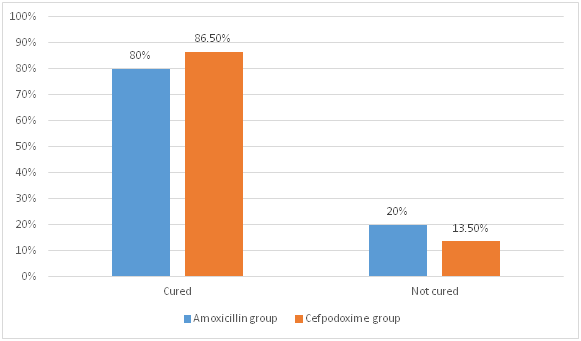
- There were no significant differences in the treatment outcomes in both groups (p =0.171) as shown in figure 1.
Figure 1. Comparison of treatment outcomes
Conclusion
- Treatment of pneumonia with amoxicillin and cefpodoxime resulted in a similar response to therapy and the difference was not statistically significant.
- Both the groups showed a significant improvement in mean body temperature, respiratory rate and wheeze.
Bangladesh J Infect Dis 2019; 6(1): 22-25.
Related Topics