Background
Inhaled short-acting ?2-agonists (SABA) are often the mainstay of treatment for symptom relief in mild asthma patients. Such patients are poorly adherent to maintenance therapy and often underuse inhaled glucocorticoids. This is one of the main reasons for severe asthma exacerbations in such patients. Treating such patients with a fast-acting reliever in combination with an inhaled glucocorticoid component on an ‘as-needed’ basis is yet another potential strategy that can alleviate symptoms and exacerbation risk.
Aim
To determine whether as needed use of budesonide–formoterol would be non-inferior to regular budesonide maintenance treatment in preventing severe exacerbations in patients with mild asthma.
Patient Profile
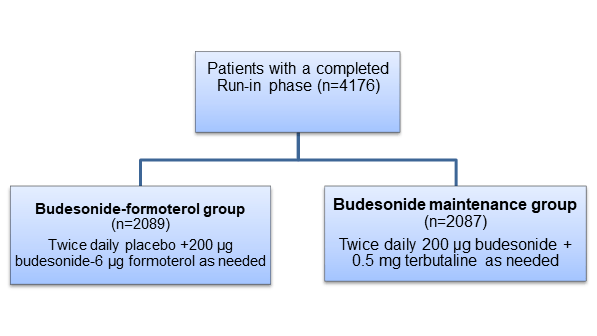
- Patients with a clinical diagnosis of mild asthma for at least 6 months before the trial initiation (age ≥12 years, n=4176)
- All patients required treatment with regular low-dose inhaled glucocorticoids (GINA step 2 treatment)
Exclusion Criteria
- Patients with worsening asthma that required a change in asthma treatment and had used systemic glucocorticoids in the previous 30 days
- Current or former smokers with a history of 10 or more pack-years
- Patients with a history of life-threatening asthma
Methods
Study Design
- Double-blind, randomized, multicenter, parallel group, phase-3 trial
Treatment
Run-in Phase
- Duration: 2-4 weeks
- Patients were treated with terbutaline 0.5 mg, as needed for symptoms
- Patients using terbutaline as needed on at least 3 days during the last week of the run-in phase and having used less than six inhalations of as-needed terbutaline per day for at least 2 days of 14 days in the run-in phase were included in the randomization phase.
Randomization Phase
Endpoints
Primary Endpoint
- Annualized rate of severe exacerbations (prespecified non-inferiority limit of 1.2) in ‘as needed’ budesonide-formoterol group and budesonide maintenance group
Secondary Endpoints
- Time to the first severe exacerbation and use of inhaled and systemic glucocorticoids
- Use of maintenance therapy and as-needed reliever therapy
- The percentage of reliever-free days
- Score on the Asthma Control Questionnaire–5 (ACQ-5)
- Score on the standardized Asthma Quality of Life Questionnaire (AQLQ)
Trial Duration
- 52 weeks
Results
- Use of as needed budesonide–formoterol was non-inferior to budesonide maintenance therapy for severe exacerbations.
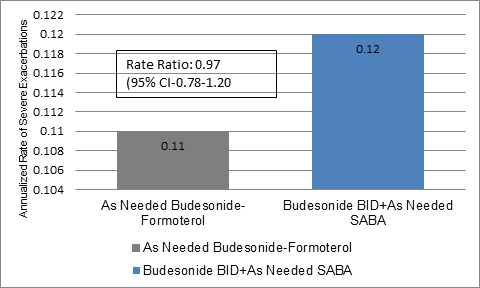
- The annualized rate of severe exacerbations was 0.11 (95% confidence interval [CI], 0.10 to 0.13) for budesonide-formoterol and 0.12 (95% CI, 0.10 to 0.14) with budesonide maintenance (rate ratio, 0.97; upper one-sided 95% confidence limit, 1.16) (Figure 1).
- The rate of severe exacerbations resulting in emergency department visit or hospitalization was similar in both the study groups.
- There was no difference in time to the first exacerbation in the two study groups [hazard ratio (HR), 0.96; 95% CI, 0.78 to 1.17].
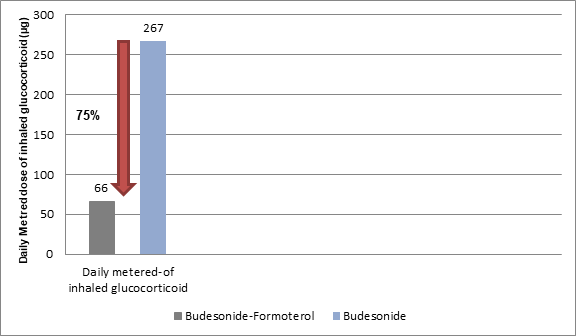
- Patients treated with budesonide-formoterol required a 75% lower median daily metered dose of inhaled glucocorticoid as compared to the budesonide maintenance group (Figure 2).
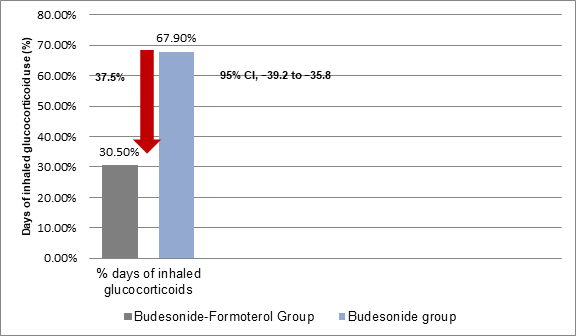
- The percentage days with inhaled glucocorticoid use was lower in patients in the budesonide–formoterol group vs. those in the budesonide maintenance group (Figure 3).
- There was no difference in the median number of days for use of systemic glucocorticoids in both the treatment groups.
- Patients in the budesonide-formoterol group vs. those in the budesonide maintenance group had fewer days with no use of as-needed agents (69% vs. 75.9%). Though the mean change from baseline in the percentage of reliever-free days was lower in the budesonide-formoterol group vs. the budesonide maintenance group, the mean change from baseline in the as-needed use of as-needed reliever treatment did not differ significantly between groups.
- The change in ACQ-5 score differed by 0.11 units (95% CI, 0.07 to 0.15) in favor of budesonide maintenance therapy.
- The change in the AQLQ overall score was less in the budesonide–formoterol group vs. the budesonide maintenance group (mean difference, −0.10; 95% CI, −0.14 to −0.05).
- Fewer patients had high use of as-needed budesonide–formoterol rather than as-needed terbutaline.
- Fewer patients in the budesonide–formoterol group rather than budesonide maintenance group required more than 8 inhalations of the as-needed agent per day (10.4% vs. 15.0%) or more than 12 inhalations per day (4.1% vs. 7.4%) at least once.
Conclusions
- Amongst patients with mild asthma, as needed use of budesonide–formoterol was non-inferior to twice-daily budesonide in terms of the rate of severe asthma exacerbations and time to first severe exacerbation during 52 weeks of treatment
- Patients treated with budesonide-formoterol achieved the rate of severe exacerbation with less than one quarter of the inhaled glucocorticosteroid dose as compared to those on budesonide maintenance therapy.
- Patients treated with budesonide–formoterol had lower exposure to the inhaled glucocorticoid as compared to those treated with budesonide maintenance group.
N Engl J Med 2018; 378: 1877-87.