ATLAS Study: Low Dose Amisulpride, an Effective Treatment Strategy for Late-Onset Schizophrenia-like Psychosis
19 Jul, 18
Background
Although very late (aged ≥60 years) onset schizophrenia-like psychosis is frequent there are no placebo-controlled, randomised trials to assess the efficacy and risks of antipsychotic treatment. Moreover, such patients usually do not have insight into the potential value of taking antipsychotic treatment, leading to lack of antipsychotic treatment.
Aim
- The ATLAS (Antipsychotic treatment of very late-onset schizophrenia-like psychosis) study aimed at determining the superiority of low-dose amisulpride (100 mg daily) vs. placebo in alleviating psychosis symptoms over 12 weeks.
- The study also determined the benefits of prolonged treatment (beyond 12 weeks).
- Side effects and serious adverse effects associated with amisulpride were also determined.
Patient Profile
- Patients aged 60 years and above suffering with very late-onset schizophrenia-like psychosis (n=101, mean age; 80.2 years, majority females)
- Very late-onset schizophrenia-like psychosis was defined as per the International Consensus Group Criteria, as onset of delusions or hallucinations, or both, at age 60 years or older
- All patients had Brief Psychiatric Rating Scale (BPRS) score of ≥30, without cognitive impairment
Methods
Study Design
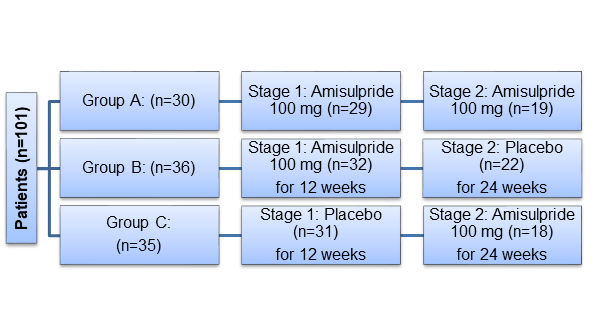
- Pragmatic, multi-centre, randomised, three-arm, placebo-controlled, double-blind trial conducted in two stages
Randomization
- Patients were randomized 1:1:1 to one of the following groups:
Outcomes
Primary Outcomes
- Change in psychosis symptoms, assessed by the BPRS score, between baseline and week 12 and between week 12 and the final assessment (week 24 or 36)
- The proportion of patients who withdrew from treatment due to a perceived absence of efficacy between baseline and week 12 and between week 13 and the final assessment
Secondary Outcome
- Extrapyramidal side-effects (as measured with the Simpson Angus Scale)
- Treatment compliance
Assessments were carried out at baseline, week 4, 12, 24 or 36
Results
- Of the 101 study subjects, 91% (n=92) took trial medication, of these, 64% (n=59) completed stage 1 and 58% from the stage 1 (n=34) completed stage 2 treatment.
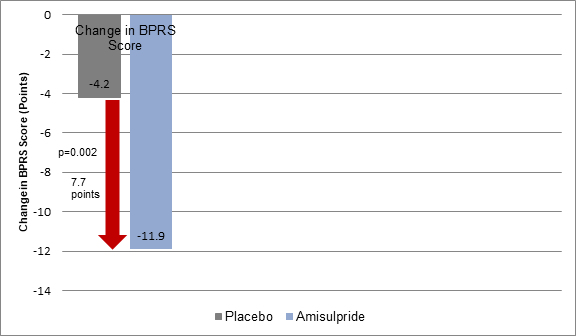
- The improvement in BPRS scores at 12 weeks was greater with amisulpride vs. placebo, despite suboptimal compliance (p=0·0002) (Figure 1).
Figure 1: Mean Change in BPRS scores in stage 1 in the study groups
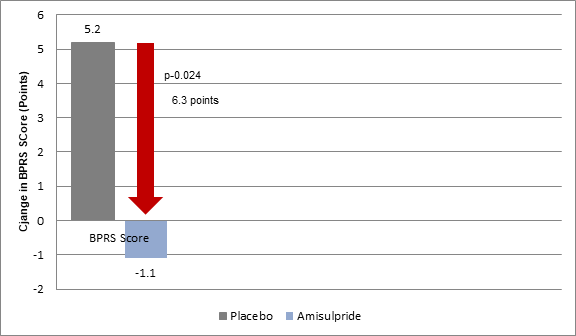
- The BPRS scores in stage 2 improved by a mean of 1·1 points from 12 weeks to the final assessment in the patients who continued amisulpride treatment but deteriorated by 5·2 points in those who were switched from amisulpride to placebo (p=0·024) (Figure 2).
Figure 2: Mean Change in BPRS scores in stage 2 in the study groups
- Greater proportion of patients assigned to amisulpride completed the treatment vs. those assigned to placebo (67% vs. 58%; p=0.39). Perceived non-efficacy of the medication in stage 1 (p=0·010) and stage 2 (p=0·031) was the main reason for treatment discontinuation.
- The incidence of serious adverse events was more frequent in the amisulpride group vs. the placebo group in stage 1 (p=0·057) and stage 2 (p=0·19). The most common serious adverse events included infection (five patients in the amisulpride group, three in the placebo group) and extrapyramidal side-effects (three patients in the amisulpride group, none in the placebo group).
- Five deaths were recorded during the study, one due to gastric ulcer bleed before treatment initiation (group B), two while taking stage 2 treatment (one in group A and one in group C), and two amongst those who stopped treatment in stage 1 and died many weeks later (one in group B and one in group C). None of the deaths were related to treatment.
Conclusion
- A single daily low-dose (100 mg) amisulpride effectively treated symptoms of very late-onset schizophrenia-like psychosis.
- The benefits can be maintained by prolonging the treatment.
Lancet Psychiatry. 2018 Jul; 5(7): 553–563.
Related Topics