Budesonide/Formoterol Combination Superior to Fluticasone/Salmeterol in Preventing Exacerbations in COPD Patients: PATHOS Study
Background
Patients with moderate and severe chronic obstructive pulmonary disease (COPD) are often treated with a combination of inhaled corticosteroids (ICSs) and long-acting ?2-agonists (LABAs). However there have been no head to head studies comparing the effects of the various ICS/LABA combinations available for managing COPD.
Aim
To compare the exacerbation rates in primary care COPD patients treated with budesonide/formoterol vs. fluticasone/salmeterol.
Patient Profile
- Patients with physician-diagnosed COPD and a follow-up of up to 11 years or end of treatment with fixed ICS/LABA combination
- All patients had a record of post-diagnosis treatment with a fixed combination of budesonide/formoterol (n=7155) or fluticasone/salmeterol (n=2378)
- Mean age; 67 years, females; 53%
Methods
Study Design
- Propensity score-matched, population-based, retrospective, observational cohort study
Outcomes
- COPD Exacerbation rates (exacerbations were defined as hospitalizations, emergency visits and collection of oral steroids or antibiotics for COPD)
Follow-up
- 3.5 years (19170 patient years)
Results
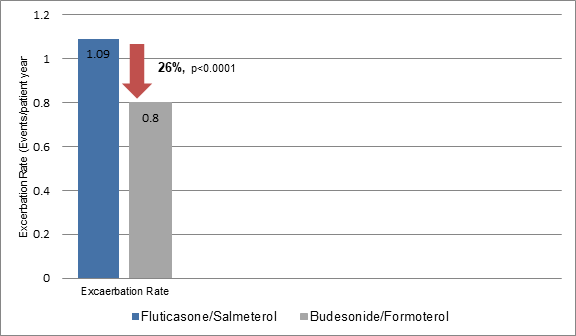
- The exacerbation rates were 26.6% lesser in budesonide/formoterol group vs. fluticasone/salmeterol group (Figure 1). Thus, 3.4 patients were needed to be treated with budesonide/formoterol vs. fluticasone/salmeterol to prevent one exacerbation per patient-year.
- Use of budesonide/formoterol was more frequent in those with less than one exacerbation annually. On the contrary, patients with one or more exacerbations annually used fluticasone/salmeterol more frequently than budesonide/formoterol.
- The mean dose of budesonide/formoterol during the study period was 568 µg/day and that of fluticasone/salmeterol was 783 µg/day.
- The annual rates for COPD-related hospitalizations were lesser by 29.1% in the budesonide/formoterol group vs. fluticasone/salmeterol group (Table 1). The number needed to treat to prevent one COPD-related hospitalization per patient-year was 16.0 with budesonide/formoterol vs. fluticasone/salmeterol.
- Patients treated with budesonide/formoterol spent 27% fewer days in hospital due to COPD exacerbations vs. those treated with fluticasone/salmeterol (Table 1).
- Patients treated with budesonide/formoterol had 21% fewer emergency visits to hospital than those treated with fluticasone/salmeterol (Table 1).
- Patients treated with budesonide/formoterol required 26% fewer courses of oral steroids and 29% fewer antibiotics as compared to patients treated with fluticasone/salmeterol (Table 1).
|
Outcome |
Fluticasone/salmeterol |
Budesonide/Formoterol |
Rate ratio |
P value |
|
COPD-related hospitalization |
0.21 days/year |
0.15 days/year |
0.71 |
< 0.0001 |
|
Days of hospitalization |
0.95 days/year |
0.63 days/year |
0.66 |
< 0.0001 |
|
No. of emergency visits |
0.034 events/patient year |
0.027 events/patient year |
0.79 |
0.0003 |
|
Need for oral steroids |
0.85 events/year |
0.63 events/year |
0.74 |
<0.0001 |
|
Need for antibiotics |
0.54 events/year |
0.38 events/year |
0.70 |
<0.0001 |
- Nearly 13.8 % of the patients treated with fluticasone/salmeterol switched to budesonide/formoterol and 2.8% of the budesonide/formoterol users switched to fluticasone/salmeterol during the study period.
- Use of additional tiotropium was 16% less in patients treated with budesonide/formoterol vs. those treated with fluticasone/salmeterol (rate ratio; 0.84, p<0.0001). Similarly, ipratropium and ?-agonist prescriptions were less frequent in patients treated with budesonide/formoterol vs. fluticasone/salmeterol.
Conclusions
- Long-term treatment with fixed combination budesonide/formoterol resulted in fewer moderate to severe exacerbations as compared to the long-term use of fluticasone/salmeterol in COPD patients.
- The incidence of healthcare-related outcomes including; annual rates of hospitalization, emergency hospital visits, need for oral steroids or antibiotics, and need for additional treatment with drugs like tiotropium were lesser in the budesonide/formoterol combination as compared to fluticasone/salmeterol.
J Intern Med 2013; 273: 584–594.