Introduction
Multiple topical therapies and surgical interventions are targeted towards achieving clinical disease control in patients with chronic rhinosinusitis (CRS). High-volume corticosteroid nasal irrigation (CSNI) is gaining popularity as a treatment option in patients with CRS. In Brazil, alternatives such as 1% compounded budesonide drops, or betamethasone cream are used to optimize its cost and popularize its use. However, there is paucity of data on the efficacy of these alternative modalities and hence the need to evaluate them.
Aim
This study compares the clinical efficacy of nasal irrigation with 1% compounded budesonide drops or betamethasone cream to nasal sprays in patients with CRS.
Methods
Study Design
- Retrospective, observational study.
Patient Profile
- Adults >18 years diagnosed with CRS.
- Treatment cycles between 3 or 6 months with CSNI or corticosteroid nasal spray (CSNS)
- Complete pre- and post-treatment subjective and objective evaluation data
Treatment Strategy
- The cohort comprised of 108 patients in the CSNI group with 292 treatment cycles and 149 in CSNS group with 300 treatment cycles.
- Medical history, details of topical therapy, smoking status were recorded on recruitment.
- Sub-analyses were performed segregating the patients with or without nasal polyps, previously submitted to endoscopic sinus surgery (ESS) or not and comparing the CSNI therapies (1% compounded budesonide drops and betamethasone cream).
Endpoints
- Subjective assessment of symptom improvement
- Adverse events (AEs)
- Exacerbations
- Need for surgery
- Objective clinical assessments using 22-item sinonasal outcome test (SNOT-22) score and Lund-Kennedy endoscopic score (LKES)
Results
- There was significant improvement in the LKES with both CSNI (4.8 to 4.4; p=0.01) and CSNS (4.6 to 4.3; p=0.02).
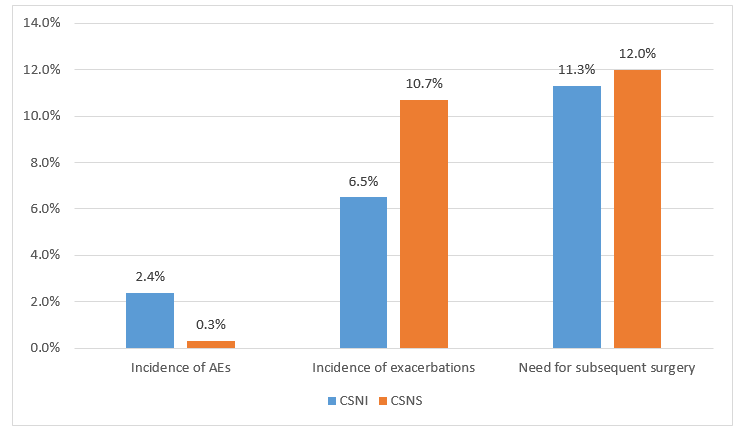
- CSNI group had more adverse events as compared to CSNS group (p=0.04) as seen in Figure 1.
- CSNI was more effective in preventing exacerbations (p=0.07), as seen in Figure 1.
- There was no statistical difference in the % of patients requiring subsequent surgery (p=0.79).
- Similar results were observed in the subgroup of patients with CRS with nasal polyps (CRSwNP). CSNI group had more AEs (2.5% vs 0.4%) but fewer exacerbations (Pr6.2% vs 10.6%).
- When the subgroup of patients with previous ESS was analyzed, CSNI treatment resulted in better subjective improvement in symptoms (85.3% vs 77.2%) and lower rate of exacerbations (6.6% 13.3%).
- The LKES improved only in the CSNI group (4.7 to 4.3).
- In the subgroup who never underwent ESS, improvement in SNOT-22 was demonstrated only by the CSNI group (p=0.04).
- Better improvement in LKES was noted with 1% compounded budesonide drops than betamethasone cream, with fewer adverse events.
- Higher dose (1,000 µg) of 1% compounded budesonide drops was more effective than 500 µg with better improvements in the SNOT-22 and LKES.
Conclusion
- Corticosteroid nasal irrigation (CSNI) with 1% compounded budesonide drops or betamethasone cream was effective in improving the Lund-Kennedy endoscopic score in chronic rhinosinusitis, especially in patients with nasal polyps and previous surgery.
- Additionally, CSNI was associated with higher rate of subjective improvement and fewer exacerbations than corticosteroid nasal spray, but with more adverse events.
- 1% compounded budesonide drops improved the nasal endoscopic condition with fewer adverse events than betamethasone cream, particularly at 1000 µg dose.
Braz J Otorhinolaryngol. 2021 Aug 4; S1808-8694(21)00131-2. Doi: 10.1016/j.bjorl.2021.06.008.