CREATE-X Trial: Adjuvant Capecitabine Safe and Effective in HER2-Negative Breast Cancer Patients
Introduction
Capecitabine is an oral prodrug of fluorouracil. Adjuvant capecitabine chemotherapy in patients with gastrointestinal cancer has been shown to be efficacious. Efficacy of capecitabine in patients with breast cancer is unknown.
Aim
Capecitabine for Residual Cancer as Adjuvant Therapy (CREATE-X) trial evaluated the efficacy and safety of adjuvant capecitabine monotherapy in patients with human epidermal growth factor receptor 2(HER2)-negative primary breast cancer who had residual invasive disease after the receipt of standard neoadjuvant chemotherapy containing anthracycline, taxane, or both.
Patient Profile
- Patients who had HER2-negative breast cancer of stage I through IIIB and pathologically assessed residual cancer cells after standard neoadjuvant chemotherapy containing anthracycline, taxane, or both
- Median age of the patients was 48 years (range, 25 to 74).
|
Characteristic |
Capecitabine Group (N = 443) |
Control Group (N = 444) |
||
|
Menopausal status — no. (%) |
|
|
|
|
|
Premenopausal |
262 (59.1) |
248 (55.9) |
||
|
Postmenopausal |
181 (40.9) |
196 (44.1) |
||
|
Body-mass index† |
|
|
||
|
Median (Range) |
22.6 (15.6 39.9) |
23.0 (15.6–41.2) |
||
|
Tumor size at diagnosis — no./total no. (%) |
|
|
||
|
≤2 cm |
68/442 (15.4) |
61/444 (13.7) |
||
|
>2 to ≤5 cm |
244/442 (55.2) |
275/444 (61.9) |
||
|
>5 cm |
65/442 (14.7) |
69/444 (15.5) |
||
|
Skin or chest-wall infiltration of any size — no./total no. (%) |
65/442 (14.7) |
39/444 (8.8) |
||
|
Hormone-receptor status — no. (%) |
|
|
||
|
Estrogen-receptor positive or progesterone-receptor positive |
304 (68.6) |
297 (66.9) |
||
|
Estrogen-receptor negative and progesterone-receptor negative |
139 (31.4) |
147 (33.1) |
||
|
Neoadjuvant chemotherapy — no. (%) |
|
|
|
|
|
Sequential anthracycline and taxane |
357(80.6) |
372 (83.8) |
||
|
Concurrent anthracycline and taxane |
63 (14.2) |
53 (11.9) |
||
|
Anthracycline-containing chemotherapy only or docetaxel and cyclophosphamide only |
23 (5.2) |
19(4.3) |
||
|
Fluorouracil plus anthracycline‡ |
262 (59.1) |
271 (61.0) |
||
|
Pathological-effect grade — no./total no. (%)§ |
|
|
||
|
0 |
19/434 (4.4) |
13/435 (3.0) |
||
|
1a or 1b |
232/434 (53.5) |
220/435 (50.6) |
||
|
2 or 3 |
183/434 (42.2) |
202/435 (46.4) |
||
|
No. of lymph nodes involved on histologic assessment — no. (%) |
|
|
||
|
0 |
176 (39.7) |
171 (38.5) |
||
|
1–3 |
165 (37.2) |
174 (39.2) |
||
|
≥4 |
102 (23.0) |
99 (22.3) |
||
|
Adjuvant endocrine therapy — no. (%) |
|
|
||
|
Yes |
298 (67.3) |
304 (68.5) |
||
|
No |
145 (32.7) |
140 (31.5) |
||
|
Radiotherapy — no. (%) |
|
|
||
|
Yes |
321 (72.5) |
326 (73.4) |
||
|
No |
122 (27.5) |
118 (26.6) |
||
Methods
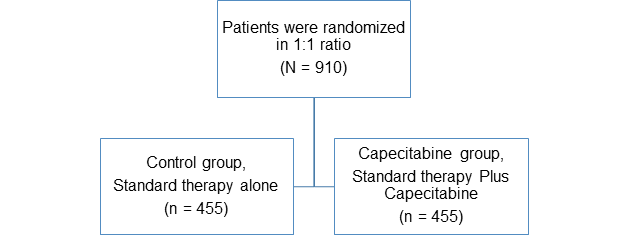
Study Design
- Multicenter
- Open-label
- Randomized
- Phase 3 trial
- N=910
Treatment
- Standard therapy included
- 5 years of endocrine therapy for hormone-receptor-positive cancer
- No further systemic treatment for hormone-receptor-negative cancer, and radiotherapy if indicated
- Endocrine therapy was administered as follows
- 5 years of tamoxifen or toremifene, combined with a gonadotropin-releasing hormone analogue as needed, in premenopausal patients or
- 5 years of aromatase inhibitors, tamoxifen, or toremifene in postmenopausal patients
- Capecitabine group: Oral capecitabine (at a dose of 1250 mg per square meter of body-surface area, twice per day, on days 1 to 14) every 3 weeks for six or eight cycles
- Control group: Standard therapy
Endpoints
- The primary endpoint was disease-free survival.
- Defined as the time from randomization to recurrence, the development of a second cancer, or death from any cause.
- Secondary endpoints included overall survival
- Defined as the time from randomization to death from any cause
Results
- Patients in capecitabine group showed longer disease-free survival than in the control group
- 74.1% vs. 67.6% of the patients were alive and free from recurrence or second cancer at 5 years (hazard ratio for recurrence, second cancer, or death was 0.70; P = 0.01)
- Overall survival was longer in the capecitabine group than in the control group
- 89.2% vs. 83.6% of the patients were alive at 5 years; hazard ratio for death, 0.59; P = 0.01)
- In patients with hormone receptor negative (triple negative )disease,
- The rate of disease-free survival was 69.8% in the capecitabine group versus 56.1% in the control group (hazard ratio for recurrence, second cancer, or death, 0.58)
- The overall survival rate was 78.8% versus 70.3% (hazard ratio for death, 0.52)
- The most common adverse reaction reported was hand-foot syndrome that occurred in 73.4% of the patients in the capecitabine group
- Other common adverse events reported were myelotoxic effects, hepatic dysfunction, and gastrointestinal symptoms
Conclusion
- Addition of adjuvant capecitabine therapy after standard neoadjuvant chemotherapy containing anthracycline, taxane, or both was safe and effective in patients with HER2- negative breast cancer who had residual invasive disease on pathological testing
- Adjuvant capecitabine therapy prolonged disease-free survival and overall survival among patients with breast cancer who had a poor prognosis, including those with triple-negative disease
N Engl J Med 2017;376:2147-59. DOI: 10.1056/NEJMoa1612645