Introduction
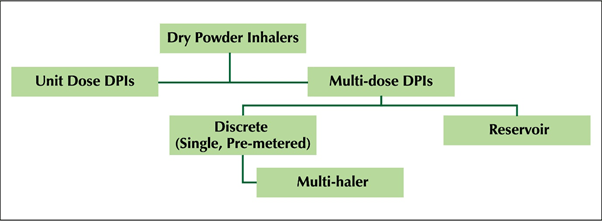
- Dry powder inhalers (DPIs) dispense medication in a dry powder formulation and are breath-activated devices.
- The inhalation technique is different from the pressurized metered dose inhalers (pMDls), as the DPIs do not require timing and coordination which is essential with pMDls.
- In DPIs the micronized drug is mixed with large carrier particles. Lactose is the most commonly used carrier. During inhalation, the small drug particles separate from their agglomerations or from the surfaces of carrier particles and deposit in the respiratory tract.
- Many DPIs commercialized since 1987 are inherently discrete multi-dose and require reduced patient manipulation and handling time before dosing. The first multi-dose DPIs were commercialized in Europe in 1988.
- Multidose dry powder inhalers (mDPls) are easy to use, have reduced device handling errors and preferred by patients.
- The new Multi-hale TM (60 doses) is an advanced discrete mDPI (Figure 1) with an in-built dose counter containing 60 pre-loaded doses.
Design of Multi-Haler TM (60 Doses)
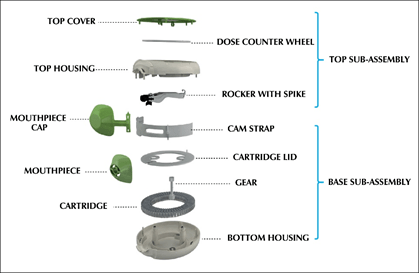
- The Multi-haler TM (60 doses) is designed to hold additional grooves at mouth-piece and fins on the spike for optimum evacuation of drug-formulation (Figure 2).
Each part of Multi-haler TM (60 doses) is manufactured to precise specifications and some of its major parts include the cartridge, gear, cam-strap, mouthpiece, spike and dose counter wheel. (Figure 3)
Working of Multi-Haler TM (60 Doses)
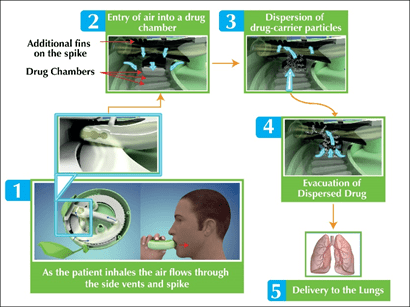
- When the slider of the new Multi-haler TM (60 doses) is pushed back, the spike is forced down on the aluminum foil of a single drug holding chamber and tears it. The device is ready for use when both the arrows meet.
- As the patient starts inhaling (Figure 4), the drug is evacuated from the single drug chamber via the mouthpiece. Air enters the device from the gaps in the side vents. Air then moves through the spike into the drug chamber. Also, the air enters the drug chamber along the fins on the spike. The air entering the chamber causes a pressure drop and the subsequent turbulence leads to the drug to be evacuated from the capsule chamber.
- The evacuated drug passes through the mouthpiece into the airways into the lungs. After completely inhaling the drug, the slider should be brought back to its initial position.
- The integrated dose counter on the top of Multi-haler TM (60 doses) will count down by one dose and display the number of doses left in the inhaler.
Performance of Multi-Haler TM (60 doses)
- Performance of a device is critical to determining optimal drug deposition in the lungs which in turn impacts treatment outcomes.
Methods
- To evaluate design and in-vitro performance of Salmeterol/Fluticasone Propionate-50mcg/250mcg (SFC-250) Multi-haler TM (60 doses). Formulation Product: Seroflo 250 Multi-haler TM (60 doses).
- The Multi-haler TM (60 doses) was evaluated for technical design parameters of delivered dose uniformity (DDU), device resistance and stability at controlled temperature & humidity. The in-vitro parameters evaluated were percent fine particle mass (%FPM) and mass median aerodynamic diameter (MMAD) at various flow rates of 30, 60 and 90 L/min.
- Three random samples of SFC-250 Multi-haler TM (60 doses); Seroflo 250, Cipla Ltd., Batch number: 00338-250/50-901116; were evaluated for the above in-vitro parameters.
- Device resistance was measured using Critical Flow Controller (TPK 2000, Copley Scientific).
- Dosage Unit Sampling Apparatus (DUSA, Copley Scientific) was used to perform DDU through the entire life of Multi-haler' TM (60 doses TM at a flow rate of 60L/min at controlled temperature and humidity. The DUSA for DPIs is used to perform those tests specified by the Pharmacopoeias that relate to "delivered" or "emitted" dose, namely "Uniformity of Delivered Dose", "DDU through container life".
- Next Generation Impactor (NGI, Copley Scientific) was used for measuring %FPM and Mass Median Aerodynamic Diameter (MMAD).
- The product was monitored on stability for aerodynamic particle size distribution, delivered dose uniformity, assay, related Substances at 25°C/60% RH, 30°C/65% RH and 40°C/75% RH conditions.
- The Multi-haler" (60 doses) devices were tested for robustness using drop test in which device was subjected to mimic device abuse scenarios.
Results
- The inspiratory resistance for Multi-haler TM (60 doses) was found to be 0.027(kPa° 5 L/min).
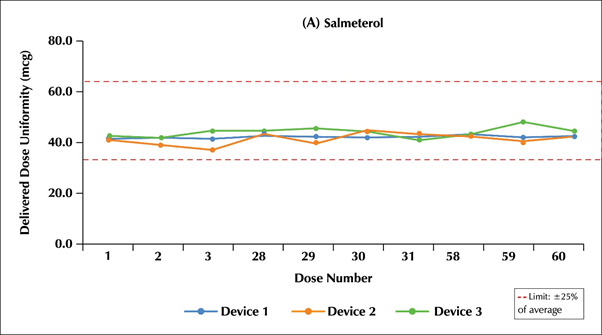
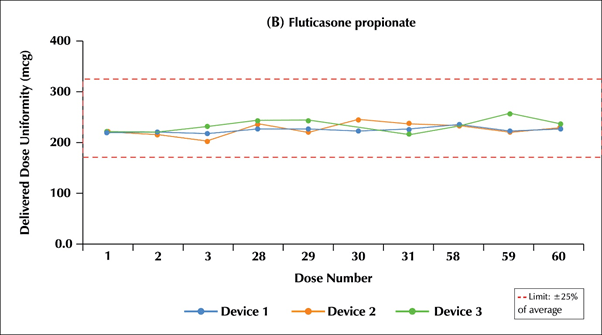
- The DDU through the entire life of Multi-haler TM (60 doses) for the salmeterol component was in the range of 93.9%-106.8% (Limit: 75.0%-125%) i.e.:46.49- 53.4 mcg (Limit: 37.5-62.5 mcg) and for the fluticasone propionate component was in the range of 92.9%-107.7% (Limit:75.0%-125%) i.e. 232.25-269.25 mcg (Limit: 189.37-312.5 mcg).
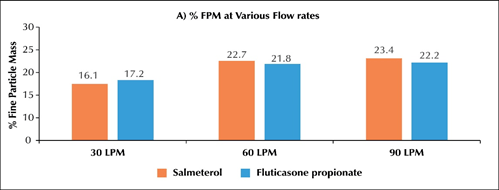
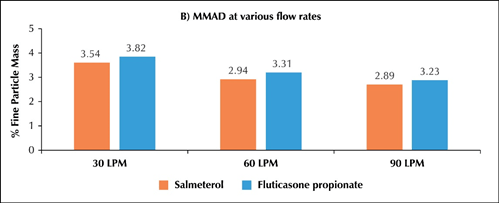
- ?In-vitro particle size distribution of Seroflo 250 Multi-haler TM (60 doses) at various flow rates
o The %FPM at 60 L/min for salmeterol was 22.66 and fluticasone propionate was 21.78 (Figure 2A).The %FPM values at 60 and 90 L/min were almost similar for both the active ingredients. At 30L/min the % FPM was slightly lower while MMAD was comparable at all flow rates
o The MMAD at 30, 60 and 90L/min for salmeterol was 3.54, 2.94 and 2.89µ while fluticasone propionate was 3.82, 3.31 and 3.23µ respectively (Figure 2B).
- Stability: The product was found to be stable with no significant change in all the mentioned three conditions.
- Robustness: The Multi-haler TM (60 doses) is a robust device and successfully withstood abuse scenarios.
Conclusion
- The Multi-haler TM (60 doses) delivered precise and consistent dosing at every dose for 60 doses and the performance was unaffected by humidity or temperature.
- It is an advanced discrete mDPI, which has reduced device handling steps and has "single action to inhalation".
- It is a robust device which withstands patient-device abuse scenarios.
- The Multi-haler TM (60 doses) was successfully tested for technical design and in-vitro parameters with SFC-250 and it complies the performance requirements of Indian pharmacopoeia for pharmaceutical-DPI products.
References
1.Respi Care 2005; 50 1209-1227
2.Med Devices (Auckl). 2015; 8: 131-139. doi: 10.2147/MDER.S48888
3.Ann Allergy Asthma Immunol 2003; 91: 55-60
4.Respiratory Medicine 2017, 123, 110-115
5.International Journal of COPD 2014:9 1 365-1 375