Introduction
Antibiotics disrupt normal gut microorganisms and this dysbiosis impairs “colonization resistance” making the subject more vulnerable to opportunistic overgrowth within the microbiota.
Aim
To examine the effects of the probiotic Saccharomyces boulardii CNCM I-745 (SB), the antibiotic Amoxicillin-Clavulanate (AC) and the combination on the microbiota and symptoms in healthy humans.
Patient Profile
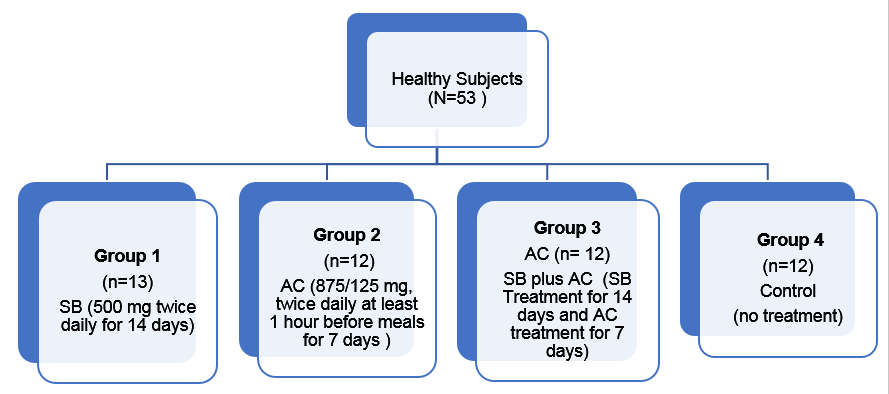
Healthy subjects with mean age of 30 years
Methods
Single-center, open-label, randomized controlled trial in healthy volunteers
- Participants gave stool samples and completed gastro-intestinal symptom questionnaires
- Microbiota changes in stool specimens were analyzed using 16s rRNA gene pyrosequencing
Endpoint
- The main endpoint was the change from baseline in the composition of the gut microbiota
- Other endpoints were Gastrointestinal Symptom Rating Scale (GSRS) scores and bowel movements characteristics recorded in a Daily Stool Log (frequency, consistency)
Results
- Significant microbiota changes were noted in the AC alone group during antibiotic treatment
- AC associated changes included reduced prevalence of the genus Roseburia and increases in Escherichia, Parabacteroides, and Enterobacter.
- Amoxicillin-Clavulanate was administered in conjunction with S. boulardii the increase in Escherichia prevalence was substantially less (0.037%, not statistically significant).
- After treatment the prevalence of Escherichia fell to baseline in both the antibiotic alone and the antibiotic with S. boulardii groups
- Microbiota alterations reverted toward baseline, but were not yet completely restored 2 weeks after antibiotic therapy
- In SB group, no significant changes in bacterial genera were noted
- Adding SB to AC led to less pronounced microbiota shifts including less overgrowth of Escherichia and to a reduction in antibiotic-associated diarrhoea scores
- Control subjects had a stable microbiota throughout the study period
- The group of subjects treated by antibiotics alone (AC group) showed worsening of the gastrointestinal symptoms during the treatment period (Days 0 to 7) (Table1)
|
Gastrointestinal Symptom Rating Scale – Scores |
Group 1 Sb (N = 13) |
Group 2 AC (N D 12) |
Group 3 Sb C AC (N = 12) |
Group 4 Control (N =12) |
Total (N = 49) |
|
Gastrointestinal symptoms total score D-7 to D0 | |||||
|
N |
13 |
12 |
12 |
0 |
37 |
|
Mean |
18.7 |
18.7 |
17.3 |
NA |
18.2 |
|
Gastrointestinal symptoms total score D0 to D7 | |||||
|
N |
13 |
12 |
12 |
12 |
49 |
|
Mean |
23.2 |
|
18.1 |
19.3 |
|
|
Gastrointestinal symptoms total score D7 to D14 | |||||
|
N |
12 |
11 |
12 |
12 |
47 |
|
Mean |
19.3 |
20.3 |
16.8 |
16.4 |
18.1 |
|
Gastrointestinal symptoms total score D14 to D21 | |||||
|
N |
12 |
11 |
12 |
12 |
47 |
|
Mean |
19.3 |
18.4 |
16.5 |
15.8 |
|
- All of the Gastrointestinal Symptom Rating Scale - Scores (GSRS) subscores from the SBCAC group were numerically lower than those from the SB alone group and from the AC group
- The most substantial and statistically significant differences were related to the “diarrhea-GSRS sub-score”
- The mean diarrhea- GSRS sub-score was statistically increased in the AC group on Day 7 compared with Day 0 (difference: 3.83 +1.10, p = 0.001)
- In inter-group comparison, the mean diarrhea- GSRS sub-score for the AC group at day 7 was significantly elevated compared with the SBCAC group (difference: 3.92 + 1.37 (p= 0.01)
- 14 of the 49 subjects (28.6%) reported adverse events (AEs) most of which were mild to moderate and related to gastrointestinal disorders including bloating, loose bowel movements and flatulence.
- S boulardii + AC group showed fewer AEs and tolerated the study regimen better than those receiving the AC alone
Conclusion
- Antibiotic treatment is associated with marked microbiota changes with both reductions and increases in different genera.
- S. boulardii administered together with amoxicillin-clavulanate, microbiota changes, including Escherichia overgrowth, were lessened and antibiotic-associated diarrhea was prevented.
- S. boulardii treatment can mitigate some antibiotic-induced microbiota changes (dysbiosis) and can also reduce antibiotic-associated diarrhea
Reference
Gut Microbes. 2017 Jan 2;8(1):17-32.