Introduction
Odontogenic infections are one of the most prevalent diseases worldwide and the principal reason for seeking dental care. Dental prescriptions account for nearly 7% to 11% of all common antibiotic prescriptions. The primary treatment in acute odontogenic infections is surgical drainage while antibiotics are an adjunct in patients showing signs of systemic involvement.
Aim
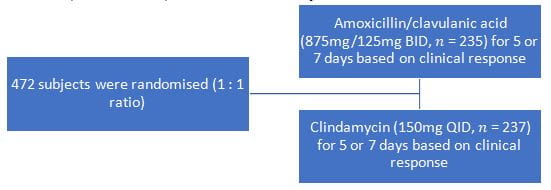
To evaluate efficacy and safety data to support twice daily dosing of amoxicillin/clavulanic acid compared to clindamycin in odontogenic infections
Patient Profile
Patients with ≥18 years of age with a diagnosis of acute odontogenic infections (periapical abscess, acute periodontitis, and pericoronitis) that required antibiotic therapy
Methods
- Phase IV, randomised, observer blind study
Study Endpoints
- The primary endpoint was percentage of subjects achieving clinical success (composite measure of pain, swelling, fever, and additional antimicrobial therapy required) at the end of treatment
- The secondary endpoints of the study included percentage of subjects achieving clinical success at Day 5 and change in the VAS score for pain and swelling from baseline to Days 2, 5, and 7
Results
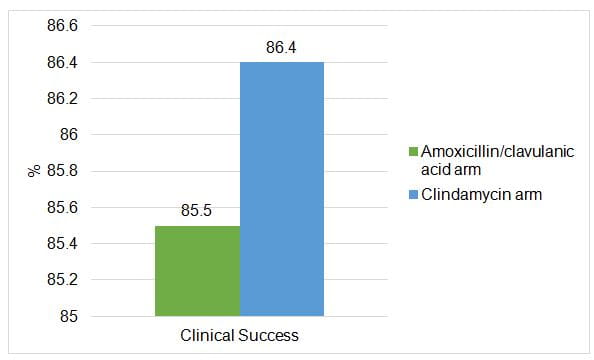
- Clinical efficacy of amoxicillin/clavulanic acid was noninferior to clindamycin
- Secondary efficacy results showed a higher clinical success rate at Day 5 in the amoxicillin/clavulanic acid arm.
- VAS by visit and treatment arms demonstrated that higher mean percentage reduction in pain by Day 2
|
|
Amoxicillin /Clavulanic |
Clindamycin |
|
VAS Score for pain |
|
|
|
Day 2 |
3.34 |
3.07 |
|
Day 5 |
5.49 |
5.38 |
|
Day 7 |
6.38 |
6.34 |
|
Mean percentage reduction in pain by Day 2 |
49.5% |
45.6% |
|
Mean percentage reduction in swelling by Day 2 |
43.6% |
39.6% |
Microbiological Results
- A total of 61 isolates were obtained from 56 samples,
- 26 in the amoxicillin/clavulanic acid arm
- 35 in the clindamycin arm
- Organisms isolated in both the treatment arms were similar and predominantly viridans streptococci group ((n= 24) including Streptococcus oralis,Streptococcus mitis, and Streptococcus parasanguinis), Enterobacter spp. (n= 10), Klebsiella spp. (n= 9), Pseudomonas spp. (n= 7), and Staphylococcus spp. (n= 5)
Safety
- Most adverse events (raised liver enzymes, diarrhoea, and headache) were similar across both arms and were of mild to moderate intensity
- The incidence of treatment emergent Adverse events (TEAEs) was similar between treatment arms, except for diarrhoea and headache which were reported in slightly more patients in the clindamycin arm
|
MedDRA preferred term |
Amoxicillin/clavulanic acid (n=236) |
Clindamycin (n=235) |
|
Total number of treatment emergent AEs |
243 |
236 |
|
Subjects who experienced at least one AE |
123 (52.1%) |
124 (52.8%) |
|
Abdominal discomfort |
11 (4.7%) |
7 (3.0%) |
|
Alanine aminotransferase increased |
26 (11.0%) |
24 (10.2%) |
|
Aspartate aminotransferase increased |
24 (10.2%) |
20 (8.5%) |
|
Blood bilirubin increased |
12 (5.1%) |
13 (5.5%) |
|
Diarrhoea |
19 (8.1%) |
28 (11.9%) |
|
Dizziness |
18 (7.6%) |
14 (6.0%) |
|
Headache |
8 (3.4%) |
14 (6.0%) |
|
Increased appetite |
20 (8.5%) |
15 (6.4%) |
|
Nausea |
7 (3.0%) |
4 (1.7%) |
|
Somnolence |
19 (8.1%) |
17 (7.2%) |
Conclusion
- Amoxicillin/clavulanic acid was comparable to clindamycin in achieving clinical success in acute odontogenic infections
- It was also found to be well tolerated with a safety profile consistent with the known pharmacologic effects of amoxicillin/clavulanic acid and with that described in the global prescribing information
Reference
Int J Dent. 2015; 2015: 472470