Efficacy and Safety of Cefixime in Management of URTI Patients
11 Jul, 22
Introduction
Cefixime is a third-generation cephalosporin with long half-life, broad spectrum of antibacterial action, safe, with speedy relief of symptoms of upper respiratory tract infections (URTI)
Aim
To evaluate the effectiveness and safety of cefixime 400 mg per day in URTI patients
Patient Profile
- Patients with upper respiratory tract infections (URTI)
Methods
- Retrospective, multicentre, observational cohort analysis
- The study enrolled 200 patients prescribed cefixime for URTI at 4 centres across India
- A prevalidated questionnaire was designed to assess the efficacy and tolerability of cefixime in the management of URTI
Endpoint
- The primary endpoint was the assessment of clinical response in terms of change in mean WURSS 21
- Secondary endpoints were the assessment of:
- Change in mean WURSS 21 Symptom score from baseline to day 5
- Change in mean WURSS 21 Quality of life score from baseline to day 5
- Change in Global severity of Patient Health condition
- Percentage of patients who discontinued the treatment
- Adverse events (AE) reported during the entire course of the therapy
Results
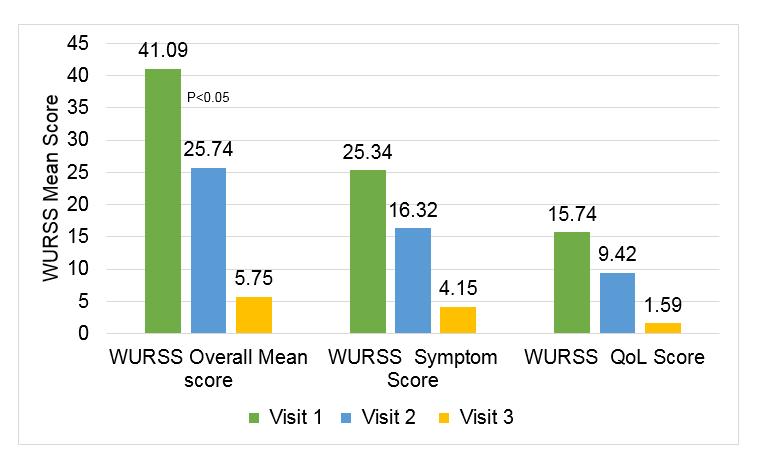
- The WURSS21 score showed significant improvement in overall mean scores in all visits after start of the treatment
Figure 1: Improvement in WURSS 21 score
- Number of patients with moderate to severe disease at visit 1 were 46 (23%), which were reduced to 5 (2.5%) with moderate disease at visit 2 and only 1 patient with moderate disease at visit 3
- 98% of the patients were improved symptomatically at visit 3 as compared to visit 1
- None of the patients reported adverse effects with cefixime therapy
Conclusion
The study confirms the effectiveness and safety of short course of cefixime therapy in URTI.
Reference
J Otorhinolaryngol Allied Sci 2020;3(3):81–85