Efficacy of 6 Months of Capecitabine vs. 12 Months as Adjuvant Chemotherapy for Patients with Stage III CRC
14 Oct, 20
Background
Surgery is the main treatment for colorectal cancer (CRC), while postoperative adjuvant chemotherapy is used to reduce recurrence and improve prognosis. The optimal treatment period for oral 5-FU drugs as the adjuvant postoperative chemotherapy for CRC remains inconclusive.
Aim
To compare 6 months vs. 12 months of capecitabine as adjuvant chemotherapy for patients with stage III CRC
Patient profile
- Patients with
- Histologically confirmed stage III colon adenocarcinoma
- curatively resected with extended lymph node dissection (D2 or D3 in the Japanese Classification of Colorectal Carcinoma, 7th edition);15
- aged 20–79 years
- Eastern Cooperative Oncology Group performance status (ECOG-PS) of 0–1
- no prior chemotherapy or radiotherapy for CRC
- no other active malignancies
- adequate oral intake
- preserved major organ functions
- no uncontrollable severe infection
Methods
- Multi-institutional, open-label, randomised, phase III study
Study Endpoints
- The primary endpoint was disease-free survival (DFS)
- Secondary endpoints were relapse-free survival (RFS), overall survival (OS), and adverse events
Results
Efficacy
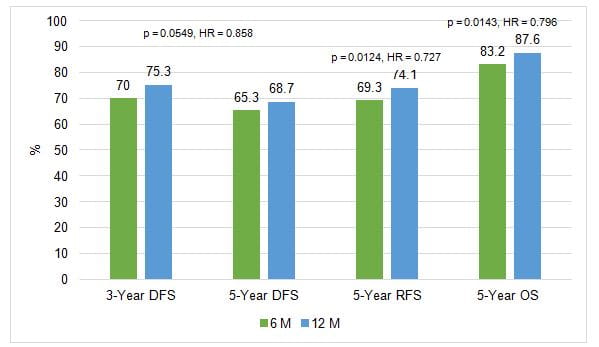
- 12 months capecitabine did not show superiority to 6 months regimen in terms of DFS
- However, 12 months capecitabine showed significant superiority to 6 months both in RFS and OS
Figure 1. Efficacy analysis: Disease-free survival and relapse-free survival and overall survival
- The difference in the hazard rate between the two groups from year 0.5 to 1.5 was statistically significant (HR = 0.713; p = 0.0149)
- The subgroup analysis showed a reduced risk of recurrence in the 12M group compared to the 6M group, particularly in the male sex, open surgical approach, and the T4 subgroup
Safety
- The incidence of overall grade 3–4 adverse events was almost comparable in both groups
- Incidence rates of adverse events in 6M was 91.7% and 94.7% in 12 M groups.
- Hand-foot syndrome (HFS) was the most commonly reported AE
- In 12 M group, the incidence of HFS was higher than in 6M group
Conclusions
- The study demonstrated that 12-month adjuvant capecitabine did not demonstrate superior DFS to that of 6-month however, OS and RFS was statistically higher in the 12M group
- The study researchers suggested 12 months of capecitabine monotherapy, along with 3 months of CAPOX with limited neurotoxicity, could be proposed as a treatment option without neurotoxicity
Reference
British Journal of Cancer.2019;120:689–696
Related Topics