EUCLID: Clopidogrel & Ticagrelor Similar in Symptomatic PAD Patients
Introduction
Peripheral artery disease (PAD), a manifestation of systemic atherosclerosis, is associated with adverse cardiovascular (CV) and limb events. The existing evidence indicates that clopidogrel is more effective than aspirin in alleviating the risk of CV events in patients with PAD. Ticagrelor, a newer antiplatelet agent, is known to be superior to clopidogrel in patients with acute coronary syndrome (ACS) or stable coronary artery disease (CAD). However, evidence on efficacy of ticagrelor specifically in PAD patients is lacking.
Aim
The EUCLID (Examining Use of Ticagrelor in Peripheral Artery Disease) trial compared the efficacy of ticagrelor vs. clopidogrel in preventing CV death, myocardial infarction (MI), or ischemic stroke in patients with symptomatic PAD.
Patient Profile
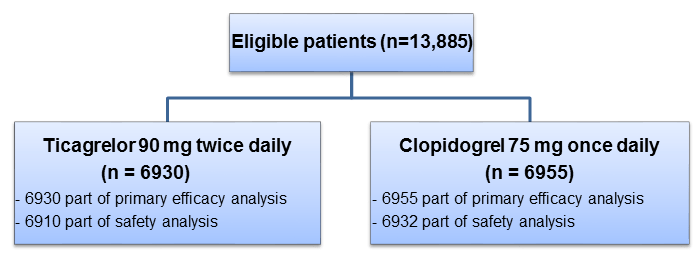
- Patients with symptomatic PAD (n=13,885; age ≥50 years; from 811 centers across 28 countries) who had a history of revascularization of the lower limbs for symptomatic disease, more than 30 days before randomization or who had hemodynamic evidence of PAD (ankle–brachial index [ABI] ≤0.80 at screening and <0.85 during randomization visit)
Method
Study Design
- Double-blind, active comparator, randomized trial
Treatment Strategy
Duration of Follow-up
- 30 months (Median duration)
Primary Efficacy Endpoint
- First occurrence of any event in the composite of CV death, MI, or ischemic stroke
Primary Safety Endpoint
- Major bleeding (as per the Thrombolysis in Myocardial Infarction [TIMI] criteria)
Key secondary Efficacy Endpoint
- Composite of primary outcome and acute limb ischemia leading to hospitalization
Results
- Of the entire study population, 43% were enrolled based on an abnormal ABI and 57% on the basis of previous revascularization.
- Most of the patients (76.6%) had claudication, 4.6% had critical limb ischemia, and 18.7% were asymptomatic. The mean baseline ABI was 0.71.
- The primary efficacy endpoint occurred in 10.8% patients receiving ticagrelor vs. 10.6% patients receiving clopidogrel (hazard ratio [HR: 1.02; p = 0.65) (Figure 1)
- Amongst the components of the primary efficacy outcome, only the incidence of ischemic stroke was slightly higher with clopidogrel vs. ticagrelor (2.4% vs. 1.9%; HR: 0.78; p=0.03)
- Other efficacy outcomes were similar among ticagrelor and clopidogrel groups (Table 1)
|
Efficacy outcomes |
Ticagrelor |
Clopidogrel |
Hazard Ratio |
P value |
|
Composite of CV death, MI, ischemic stroke, or acute limb ischemia requiring hospitalization |
12.1% |
12.0% |
1.02 |
0.74 |
|
Death from any cause |
9.1% |
9.1% |
0.99 |
0.91 |
|
Composite of CV death, MI, or ischemic/ hemorrhagic stroke |
11.1% |
10.9% |
1.02 |
0.72 |
|
Hospitalization for acute limb ischemia |
1.7% |
1.7% |
1.03 |
0.85 |
|
Lower-limb revascularization |
12.2% |
12.8% |
0.95 |
0.30 |
|
Composite of coronary or peripheral revascularization, including limb, mesenteric, renal, carotid, or other type |
17.5% |
18.0% |
0.97 |
0.46 |
- The primary safety endpoint of TIMI major bleeding did not differ for both the study groups (1.6% vs 1.6%; HR: 1.10; p = 0.49).
- The incidence of intracranial bleeding (0.5% vs 0.5%), fatal bleeding (0.1% vs 0.3%) and TIMI minor bleeding (1.2% vs 1.0%) were also similar in both the study groups.
- Adverse events leading to discontinuation were higher with ticagrelor vs. clopidogrel (15.4% vs. 11.1%): which included higher incidence of dyspnea (4.8% vs 0.2%; p<0.001) and any bleeding (2.4% vs 1.6%; p<0.001) with ticagrelor vs clopidogrel
Conclusions
In patients with symptomatic PAD,
- Ticagrelor was not superior to clopidogrel in terms of reducing CV events in patients with symptomatic PAD
- The incidence of major bleeding was similar with both clopidogrel and ticagrelor
- The rate of discontinuation was higher with ticagrelor vs. clopidogrel primarily due to dyspnea and minor bleeding events
New Engl J Med. 2017; 376:32-40.