Fixed-Dose Combination of Timolol-Dorzolamide: Comparing the Efficacy and Safety of Thrice Daily vs. Twice Daily Regimen in POAG Patients
Introduction
Fixed combination (FC) of timolol 0.5%-dorzolamide 2.0% is the most preferred FC medication for treating glaucoma. This FC brings about better reduction in intraocular pressure (IOP) as compared with the monotherapy with the individual agents and is as safe and effective as concomitant use of timolol and dorzolamide. Several ophthalmologists prefer a thrice a day timolol/dorzolamide FC administration instead of the advocated twice-daily regimen. Increasing the dosage of timolol from twice to thrice a day brings about greater reduction in IOP, though the incidence of systemic adverse events (AEs) remains a major concern.
Aim
To compare the therapeutic efficacy and safety of timolol/dorzolamide FC in patients with newly diagnosed primary open-angle glaucoma (POAG)
Patient Profile
- Patients with newly diagnosed bilateral POAG who had not received any glaucoma treatment (n=33)
Methods
Study Design
- A prospective, interventional case series
Treatment Strategy
- The study participants were initially treated with timolol/dorzolamide FC twice a day (BID; administered every 12 hours) for one month and then switched to three times a day (TDS; administered every 8 hours) for another month.
Assessments
- Patients underwent comprehensive ophthalmic examination, diurnal IOP, blood pressure (BP), and 24-h heart rate (HR) measurements at baseline, month 1 (BID), and month 2 (TDS).
Outcomes
Primary Outcome
- Change in IOP
Secondary Outcomes
- Change in HR
- Change in BP
Results
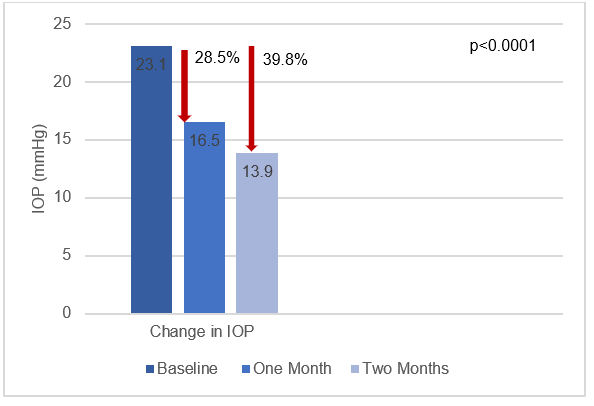
- The final analysis included 31 patients. The mean baseline IOP was 23.1 mmHg.
- Compared to baseline, the IOP decreased significantly by 28.5% at one month (16.5 mmHg) and by 39.8% at two months of follow-up (13.9 mmHg; p for both visits <0.0001) (Fig 1). The IOP decrease at month two was significantly lower that that seen at month one (p=0.0004).
- The mean change in 24-h systolic BP (SBP), diastolic BP (DBP) during the study was insignificant. The mean 24-h HR from baseline to one month was significant; though the change in 24-h HR at one and two months was comparable (Table 1).
|
Parameter |
Baseline |
One-month |
Two-month |
|
24-h SBP (mmHg) |
131.1 |
128.5 |
177 |
|
24-h DBP (mmHg) |
82.6 |
81.3 |
81.3 |
|
24-h HR (BPM) |
76.6 |
74.4* |
74.06 |
BPM: Beats per minute; DBP: Diastolic blood pressure; HR: Heartrate; SBP: Systolic blood pressure
*p=0.000
Conclusions
- In POAG patients, treatment with thrice-a-day regimen of timolol/dorzolamide FC was associated with a superior IOP-lowering effect as compared with the treatment with twice-a day regimen.
- This additional IOP lowering effect was not associated with any major systemic adverse effect or any change in heart rate and blood pressure. The safety profile of both the treatment regimens was comparable.
J Drug Assess. 2021 Aug 23;10(1):91-96.