Introduction
The incidence of ventilator-associated pneumonia (VAP) caused by multi-drug resistant (MDR) gram negative bacteria (GNB) has surged up in recent years. Paucity of effective agents against MDR GNB has resulted in re-introduction of colistin to tackle these infections. Treatment of VAP using inhaled colistin offers an attractive option as the drug achieves high concentration in respiratory tract while avoiding systemic effects. The current data on use of inhaled colistin as an adjunctive therapy for VAP in critically ill children is scarce.
Aim
To compare the safety and efficacy of inhaled plus intravenous (IV) colistin with that of IV colistin alone in critically ill children with VAP due to colistin only susceptible (COS) GNB.
Patient Profile
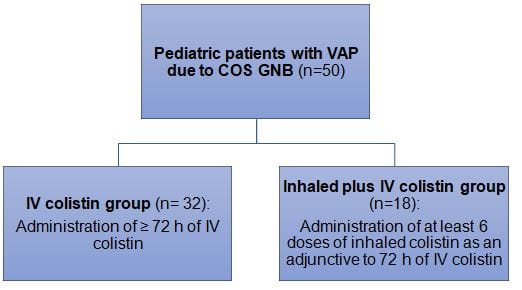
- Critically ill children (age; 1 month to 18 years) with culture-documented monomicrobial VAP due to COS GNB (n=50)
Methods
Study Design
- A retrospective cohort study
Treatment Strategy
- Patients received ≥72 h of IV colistin and at least six doses of inhaled colistin for the treatment of VAP. Based on the treatment regimen patients were categorized as:
Outcomes
Primary Outcome
- Clinical and microbiological outcomes of VAP
Secondary Outcomes
- The development of adverse events during colistin treatment
- VAP-related mortality
- Other-cause mortality
Study Duration
- 40 months
Results
- The culture and susceptibility analysis revealed the causative pathogens to be A. baumannii (n=37) and P. aeruginosa (n=13), which were resistant to all available antibiotics except colistin
- There was no evidence of development of colistin resistance in either of the study groups
- There were no between-cohort differences in clinical (p = 0.49) and microbiological outcomes (p = 0.68), bacterial eradication rate (p=0.36) or VAP-related mortality (p = 0.99).
- Addition of inhaled colistin to IV colistin had no appreciable impact on the median duration of mechanical ventilation (MV; p = 0.15) and the duration of PICU stay (p = 0.8)
- Although the bacterial eradication rates did not differ in either treatment group, the median time to bacterial eradication (TBE) was significantly shorter in the inhaled plus IV colistin group than in the IV colistin group (median of 3 days vs. median of 6 days).
- Only one patient in the IV colistin group developed reversible nephrotoxicity. Mild bronchoconstriction was observed in three patients at the time of administration of the first doses of inhaled colistin, but this did not require treatment discontinuation.
Conclusions
- This was the first, largest pediatric study that compared the safety and efficacy of inhaled plus IV colistin with that of IV colistin alone in critically ill children with VAP due to COS A. baumannii and P. aeruginosa.
- Addition of inhaled colistin to IV colistin led to a shorter TBE in critically ill children with VAP due to COS GNB.
- Nevertheless, both the treatment regimens were equally effective in terms of the clinical and microbiological outcomes of VAP.
Pediatr Drugs. 2015; 17:323–30.