To investigate whether treatment with rosuvastatin 20 mg once-daily as compared to placebo would decrease the rate of first cardiovascular events.
JUPITER Study (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin)
18 Aug, 10
JUPITER Study (Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin)
Objective
Patient Population
N=17,802; Men (>50 yrs.) and women (>60 yrs.) with no prior history of cardiovascular disease, LDL<130 mg/dL and hs-CRP≥2 mg/L.
Study Group
Patients randomized to receive either rosuvastatin 20 mg or placebo.
Study Duration
5 years (but study was stopped in 1.9 yrs. due to significant benefits with rosuvastatin).
Primary Study Endpoint
Occurrence of first major cardiovascular event, defined as nonfatal myocardial infarction (MI), nonfatal stroke, hospitalization for unstable angina, an arterial revascularization, or confirmed death from cardiovascular causes.
Results
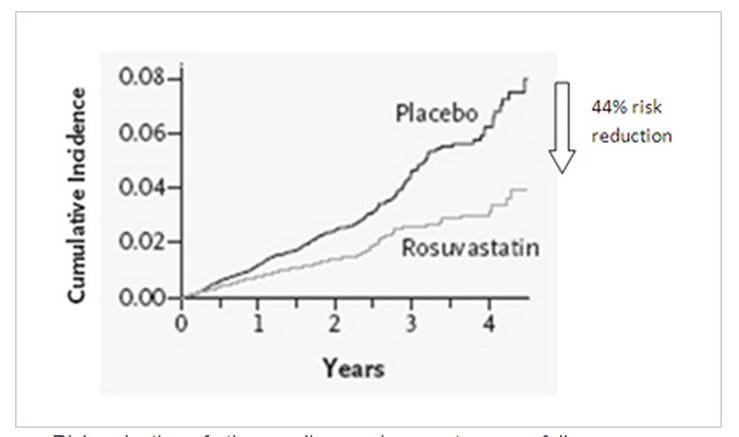
- There was a 44% risk reduction in occurrence of primary endpoint
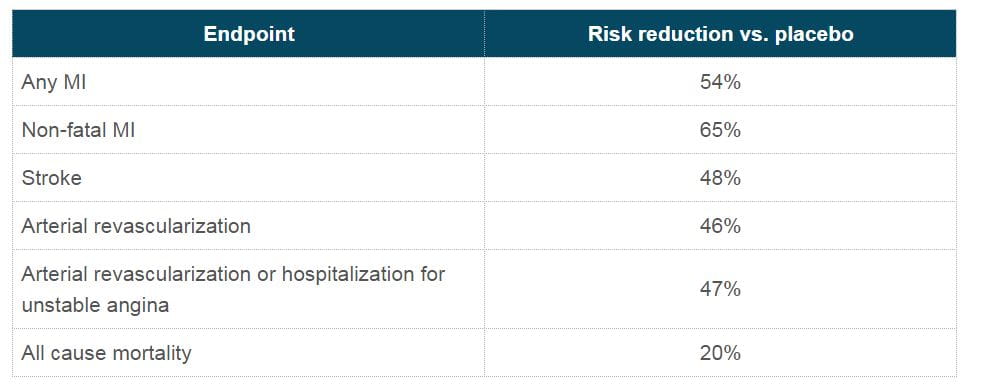
- Risk reduction of other cardiovascular events are as follows:
Conclusion
Rosuvastatin significantly reduced the incidence of major cardiovascular events in apparently healthy persons without hyperlipidemia but with elevated hs-CRP levels.
N Engl J Med 2008; 359:2195-207