Long-term Efficacy and Safety of Polyethylene Glycol as Maintenance Treatment for Childhood Functional Constipation
24 Jun, 20
Background
Functional constipation (FC) is a common pediatric problem. Guidelines recommend maintenance treatment to ensure soft, painless stools, and to prevent relapse of constipation. Nevertheless, there is a dearth of evidence to demonstrate the benefits and success of long-term maintenance treatment.
Aim
To determine the long-term efficacy of polyethylene glycol (PEG) during maintenance treatment of childhood FC
Patient Profile
- Children with FC as per the Rome III criteria (age; 2–16 years, n=115)
Methods
Study Design
- Randomized, double-blinded, single-centered, placebo-controlled trial
Treatment Strategy
- Children were randomized 1:1 to PEG (n=58) or placebo (n=57) after disimpaction
- The maintenance dose of 0.8 g/kg/day PEG was increased to maximum of 1.5 g/kg/day in case of recurrence of FC
- The medication was planned to be used for at least 8 weeks before gradually weaning it off
- Children reporting treatment failure before 24 weeks were switched to conventional treatment.
Outcomes
Primary Outcome
- Successful treatment (defined as absence of any Rome III criteria with or without use of medication after 24 weeks)
Safety Outcome
- Incidence and severity of adverse events
Follow-up Visits
- Personal visits at week 1, 2, 8 and 24
- Planned telephone consultations at week 4 and 12
Results
- The final analysis included 95 children, 47 in PEG group and 48 in placebo group.
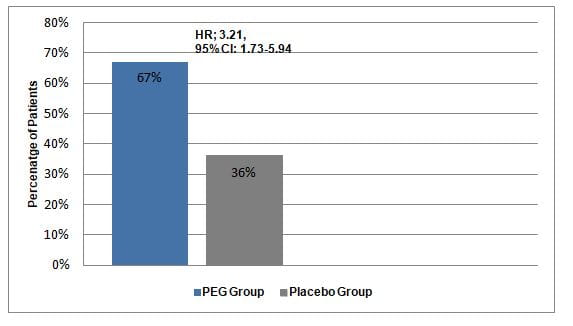
- At 24 weeks, a significantly greater proportion of patients in the PEG group were treated successfully as compared with the placebo group (hazard ratio [HR]; 3.21; 95% confidence intervals [CI],1.73–5.94) (Figure 1).
Figure 1: Treatment success in the study groups
- Significantly fewer children in the PEG group vs. the placebo group switched to rescue medication (4% vs. 57%, P<0.001).
- Time before the change to rescue medication was 13 and 27 days, respectively, for each of the 2 children in the PEG group who required rescue medication. Amongst the children in the placebo group, median time to shift to rescue medication was 27 days (range: 3–64 days).
- The incidence of fecal incontinence (FI) decreased from baseline to 24 months in PEG (from 51% to 29%) as well as in placebo group (from 44% to 26%)
- No serious adverse event related to use of the study medication were recorded during the study.
Conclusions
- Long-term maintenance treatment with PEG was significantly more effective than placebo for preventing relapse of constipation for childhood FC.
- Relapse typically occurred within the first 9 weeks after disimpaction. It is therefore recommended that maintenance treatment should commence after disimpaction.
- Long-term maintenance treatment with PEG up to 24 weeks was not associated with any significant adverse events and can be safely be used in the pediatric population.
J Pediatr Gastroenterol Nutr. 2018;67: 732–737.
Related Topics