Long-term Treatment with PPIs, Effective and Safe for Treating Eosinophilic Esophagitis in Children
24 Aug, 20
Introduction
Eosinophilic esophagitis (EOE) is a chronic disorder with increasing prevalence in children. Studies have demonstrated that nearly 50% of adult and pediatric patients with EOE respond to proton pump inhibitors (PPI). Nevertheless, there is very little data on long-term efficacy and safety of PPI treatment in the management of EOE patients, including children.
Aim
To evaluate the long-term efficacy and safety of PPIs in children with EoE
Patient Profile
- Pediatric patients with EOE; defined as the presence of ≥ 15 eosinophil/high-power field (eos/hpf) (peak value) in 1 or more biopsy samples (age 1 month to 15 years)
- Patients had at least one esophageal dysfunction symptom such as heartburn, chest pain, food impaction, abdominal pain, vomiting, regurgitation, dysphagia, and feeding difficulties
- All patients presented with histological remission to an eight-week esomeprazole trial (1 mg/kg/dose, twice daily)
- Patients had no concomitant dietary restrictions or topical steroid use
Methods
Study Design
- A prospective study conducted at two pediatric hospitals in Madrid
Treatment Strategy
- Patients were maintained on esomeprazole at 1 mg/kg/day for one year (maximum dose 40 mg/day)
Outcomes
- Symptom recurrence
- Adverse events
Assessments and follow-up
- Patients were followed-up every three months, until one year
- Endoscopy was performed at the time of clinical recurrence, worsening of symptoms, or at one year from diagnosis in cases where patients remained asymptomatic
- Adverse events were recorded at every follow-up visit
Results
- Fifty-seven children were included in this analysis (median age; 11 years, males; 73.7%).
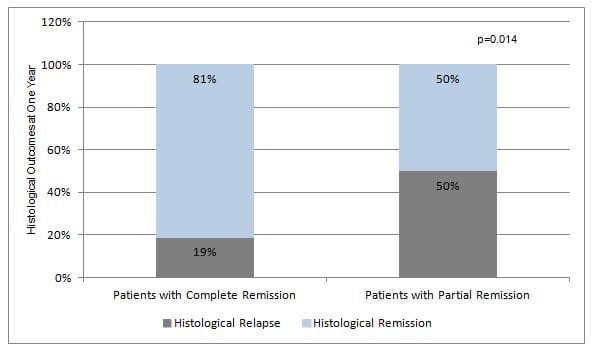
- Forty patients (70.1%; 95% CI 56.5–81.5) presented with histological remission on maintenance PPI therapy. Children with initial complete histological remission had a longer remission rate as compared with those with partial remission (81% vs. 50%, P=0.014) (Figure 1).
Figure 1: Long-term histological remission in patients
- At one year, 49 patients (86%) remained asymptomatic, 3 (5.3%) had mild symptoms and 5 patients (8.8%) had no change in symptoms.
- Pretreatment clinical and histological findings and median PPI dose/kg/day did not differ between relapsers and nonrelapsers.
- Five children experienced adverse events on PPI maintenance treatment during high-dose induction treatment. The adverse events were mild and transient and included; diarrhea, abdominal pain, urticaria or headache. There were no reports of pneumonia or diarrhea caused by Clostridium difficile.
- Eleven out of 12 children (91.6%) receiving esomeprazole 0.5mg/kg/day for additional 12 months remained in remission. Mild and transient side effects without requiring PPI avoidance were observed in 5 children.
Conclusions
- Up to 70% of children with PPI-responsive EoE remain in histological and clinical remission on a low-dose maintenance treatment at one-year follow-up, with adequate safety profile.
- Patients with complete histological remission with eight weeks of PPI treatment had a higher probability of histological remission while on maintenance therapy.
- Adverse events associated with long-term PPI treatment were infrequent, mild and transient.
J Pediatr Gastroenterol Nutr. 2018;67: 210–216
Related Topics