Introduction
Calcitonin is a physiologic endogenous inhibitor of bone resorption, decreases osteoclast formation, osteoclast attachment. The treatment with calcitonin may be beneficial in diseases associated with increased bone resorption, such as postmenopausal osteoporosis.
Aim
The Prevent Recurrence of Osteoporotic Fractures (PROOF) study determined whether salmon calcitonin nasal spray reduced the risk of new vertebral fractures in postmenopausal women with osteoporosis
Patient Profile
Postmenopausal women with osteoporosis
Methods
- The primary analysis for the incident vertebral fracture endpoint was an intention-to-treat analysis among all participants with at least one follow-up radiograph.
- Secondary analyses were performed among participants with one to five prevalent vertebral fractures at enrolment (as per protocol) and among those who received the study drug for at least 3 years or who had a fracture during the first 3 years of treatment (3-year valid completer analysis)
Results
- 200-IU dose of salmon calcitonin nasal spray was associated with significant reduction in risk of new vertebral fractures by 33%. [200 IU: 51 of 287, placebo: 70 of 270, relative risk (RR): 0.67, P = 0.03]
- The number of new vertebral fractures per 1,000 participant radiograph years was reduced by 40% (P = 0.02) in the 200-IU group compared with the placebo group
- New vertebral fractures/1000 participant radiograph years was
- Placebo =131
- 100 IU=129
- 200 IU= 78
- 400 IU=111
- In the 817 women with one to five prevalent vertebral fractures at enrollment, the risk was reduced by 36% (RR=0.64, P = 0.03).
|
|
|
Treatment Groups | ||
|
|
Placebo |
100 IU |
200 IU |
400 IU |
|
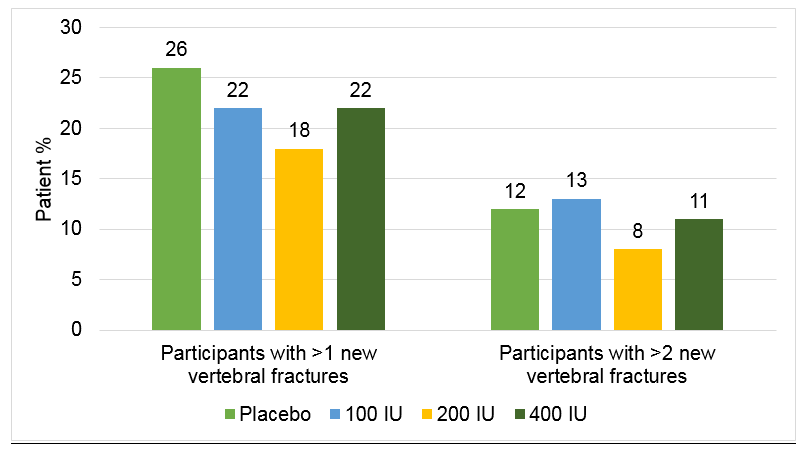
Participants with 1-5 prevalent fractures |
n =203 |
n = 201 |
n = 207 |
n =206 |
|
Participants with >1 new vertebral fractures (%) |
30 |
26 |
19 |
23 |
|
Relative risk |
|
0.94 |
0.64 |
0.78 |
|
Participants with >2 new vertebral fractures (%) |
15 |
16 |
9 |
11 |
|
Odds ratio |
|
1.09 |
0.55 |
0.73 |
|
Three-year completers |
n = 162 |
n = 152 |
n = 157 |
n = 155 |
|
Participants with >1 new vertebral fractures (%) |
36 |
32 |
26 |
27 |
|
Relative risk |
|
0.91 |
0.66 |
0.71 |
- The reductions in vertebral fractures in the 100-IU (RR = 0.85,) and the 400-IU (RR =0.84) groups were not significantly different from placebo.
- In participants receiving 100 IU salmon calcitonin nasal spray, there was a significant 64% reduction in the risk of fractures of the arm (RR=0.36, P=0.03), but the reductions in risk in the 200-IU and 400-IU groups were not statistically significant
|
|
|
Treatment Group | ||
|
|
Placebo |
Nasal Spray Salmon Calcitonin | ||
|
100 IU |
200 IU |
400 IU | ||
|
Number of participants at risk |
(n=305) |
(n = 313) |
(n =315) |
(n = 312) |
|
All nonvertebral fractures |
|
|
|
|
|
Participants % |
16 |
10 |
15 |
13 |
|
Relative risk |
|
0.64*
|
0.88
|
0.81
|
|
Hip or femoral fractures |
|
|
|
|
|
Participants % |
3 |
0.3 |
2 |
2 |
|
Relative risk |
- |
0.1* |
0.5 |
0.8 |
|
Arm fractures# |
|
|
|
|
|
Participants % |
5 |
2 |
4 |
5 |
|
Relative risk |
- |
0.4 |
0.75 |
0.84 |
*P <0.05 versus placebo.
#Fractures of the humerus, radius, ulna, or wrist.
- Significant increase in lumbar spine bone mineral density was reported in all active treatment groups (1% to 1.5%, P<0.01)
- Bone turnover was inhibited, as shown by suppression of serum type-I collagen cross-linked telopeptide (C-telopeptide) by 12% in the 200-IU group (P <0.01) and by 14% in the 400-IU group (P<0.01) as compared with placebo
- Salmon calcitonin nasal spray was well tolerated in study population
- The rate and reasons for discontinuation were distributed equally among treatment groups
- Intolerance to the nasal spray did not contribute significantly to study discontinuation; less than 5% of participants discontinued for this reason
Conclusion
- Salmon calcitonin nasal spray at a dose of 200 IU daily demonstrated significant reduction in the risk of new vertebral fractures in postmenopausal women with osteoporosis
- The findings showed it as safe and effective treatment option for postmenopausal women with established spinal osteoporosis
Reference
Am J Med. 2000; 109:267–276.