Introduction
Several therapeutic agents have been evaluated for the treatment of coronavirus disease 2019 or COVID-19. However, none of them have been proven to be efficacious. Remdesivir appeared to be promising due to its ability to inhibit SARS-CoV-2 in-vitro and reduced lung damage in nonhuman primate studies. A series of phase 3, randomized trials were conducted to evaluate the clinical efficacy of investigational agents in patients hospitalized with COVID-19. This study is the first stage of the Adaptive Covid-19 Treatment Trial (ACTT-1)
Aim
The clinical efficacy of intravenous remdesivir in hospitalized patients with Covid-19 was compared with placebo.
Methods
Study Design
- Double-blind, randomized, placebo-controlled trial
Treatment Strategy
- Adults hospitalized with Covid-19 and had evidence of lower respiratory tract infection were recruited
- The patients were randomized to receive either remdesivir (200 mg loading dose on day 1, followed by 100 mg daily for up to 9 additional days) or placebo for up to 10 days
- Patients underwent clinical assessment on an eight-category ordinal scale and the National Early Warning Score (which includes six physiological measures; total scores range from 0 to 20, with higher scores indicating greater clinical risk) daily from day 1 to day 29
- Incidence of any serious adverse events or hypersensitivity reactions was recorded
Endpoints
Primary Endpoint
- Time to recovery, defined as the first day, during the 28 days after enrollment, on which a patient met the criteria for category 1, 2, or 3 on the eight-category ordinal scale.
- The categories are as follows:
- 1, not hospitalized and no limitations of activities;
- 2, not hospitalized, with limitation of activities, home oxygen requirement, or both;
- 3, hospitalized, not requiring supplemental oxygen and no longer requiring ongoing medical care;
- 4, hospitalized, not requiring supplemental oxygen but requiring ongoing medical care;
- 5, hospitalized, requiring any supplemental oxygen;
- 6, hospitalized, requiring noninvasive ventilation or use of high-flow oxygen devices;
- 7, hospitalized, receiving invasive mechanical ventilation or extracorporeal membrane oxygenation (ECMO); and
- 8, death.
Secondary Endpoints
- Clinical status at day 15, as assessed on the ordinal scale.
- Incidence of grade 3 or 4 adverse events (AEs) or serious AEs (SAEs)
Results
- Out of the study population of 1062 patients, 541 were randomized to receive remdesivir and 521 received placebo, out of which 517 and 508 completed the trial respectively
- The baseline cohort characteristics were
- Mean age 58.9 years
- 64.5% males
- 25.9% and 54.5% had preexisting 1 or 2 comorbidities respectively
- 90.1% had severe disease at enrollment
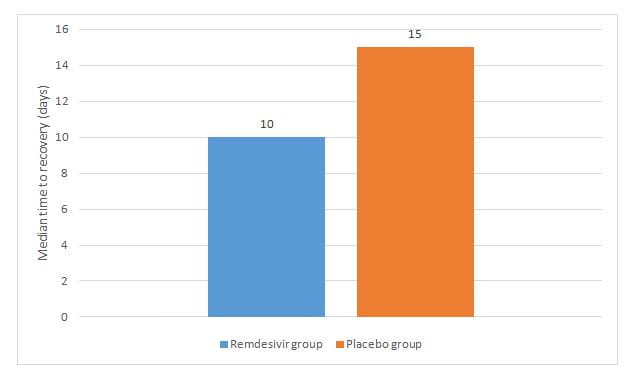
- The remdesivir group had a shorter median recovery time as compared to placebo as seen in figure 1, rate ratio for recovery, 1.29; p<0.001
- Treatment with remdesivir shoretened the time to recovery in the patients with severe disease (11 days vs 18 days), rate ratio for recovery 1.31
- Analysis using a proportional-odds model with an eight-category ordinal scale showed that the patients who received remdesivir were found to be more likely to have clinical improvement at day 15 than those who received placebo (odds ratio, 1.5; adjusted for actual disease severity)
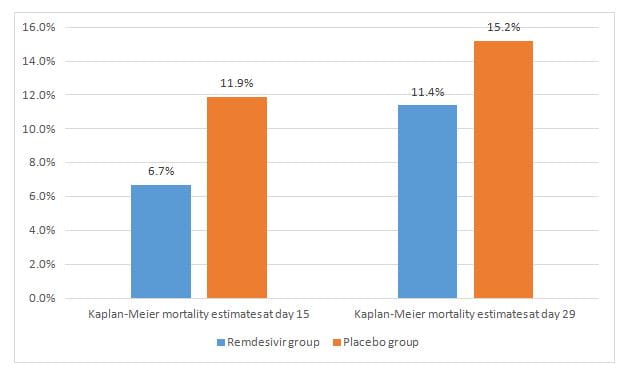
- The Kaplan–Meier estimates of mortality by day 15 and day 29 were lower in the study group (hazard ratios of 0.55 and 0.73 respectively) as seen in figure 2.
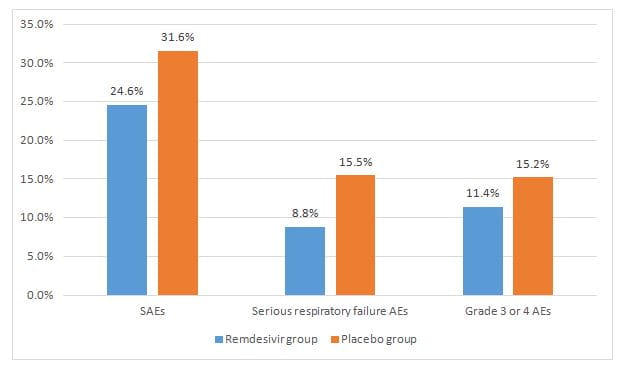
- The remdesivir group had lower incidence of SAEs, serious respiratory failure (AEs) and grade 3 or 4 AEs as compared to placebo as seen in figure 3.
Conclusion
- Treatment with remdesivir shortened the time to recovery in adults who were hospitalized with Covid-19 and had evidence of lower respiratory tract infection as compared to placebo.
N Engl J Med. 2020 Oct 8; NEJMoa2007764. Doi: 10.1056/NEJMoa2007764.