Sacubitril/Valsartan vs. Enalapril in HFrEF Patients: Analyzing the Renal Impact and Associated Outcomes
16 Aug, 18
Background
Patients with heart failure with reduced ejection fraction (HFrEF) frequently have impaired renal function, which may deteriorate further after blockade of the renin–angiotensin system. Although the combined angiotensin receptor neprilysin inhibitor (ARNI), sacubitril/valsartan reduced the risk of death and hospitalization as compared to enalapril in HFrEF patients, it did not decrease the estimated glomerular filtration rate (eGFR) by ≥50% or by >30 ml/min/1.73m2 from baseline (pre-specified secondary endpoint). Neither did it retard the progression of end stage renal disease (ESRD).
Aim
To evaluate the renal effects of sacubitril/valsartan vs. enalapril in HFrEF patients
Patient Profile
- Patients (age ≥18 years; n=8399) with New York Heart Association (NYHA) class II to IV symptoms having an ejection fraction of ≤40% (changed to ≤35% by protocol amendment) and plasma B-type natriuretic peptide (BNP) ≥150 pg/mL (or N-terminal pro-BNP [NTproBNP] ≥600 pg/mL)
- Patients with lower levels of natriuretic peptides (BNP ≥100 pg/ml or NTproBNP ≥400 pg/ml) were eligible only if they had been hospitalized for HF within 12 months.
- Patients on ongoing therapy were required to tolerate ACE inhibitor or ARB equivalent to at least enalapril 10 mg daily for at least 4 weeks before screening along with stable doses of a ?-blocker (unless contraindicated or not tolerated) and a mineralocorticoid antagonist (if indicated).
Exclusion Criteria
- Patients with symptomatic hypotension or an SBP <100 mmHg at screening or <95 mmHg at randomization
- Patients with an eGFR of <30 ml/min/1.73 m2 at screening or randomization [or a decrease >25% (amended to >35%) between screening and randomization
- Patients with hyperkalemia (serum potassium >5.2 mmol/l at screening or >5.4 mmol/l at randomization
Methods
Study Design
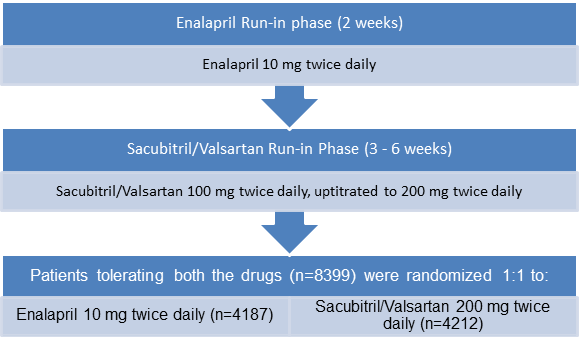
- Multi-center, randomized, double blind trial with a single blind run-in period
Treatment Strategy
Assessments
- The eGFR was estimated at screening, randomization, at 2, 4, and 8 weeks, and 4 months post-randomization; and every 4 months thereafter.
- The urinary albumin/creatinine ratio (UACR) was determined at screening, randomization, and at 1 and 8 months post-randomization.
Outcomes
Prespecified Renal Outcomes
- Time to first occurrence of any of the following:
- A 50% decline in eGFR relative to baseline
- >30 to <60 ml/min/1.73 m2 decline in eGFR relative to baseline
- Reaching ESRD
Additional (Conventional) Renal Outcomes
- Composite outcome of either a 50% decrease in the eGFR from baseline or reaching ESRD
Pre-specified Subgroup Analysis Outcome
- Occurrence of the primary renal outcomes in patients with and without chronic kidney disease (CKD)
Results
- The data on eGFR was available for all patients, and that on UACR was available in 1872 patients, at screening, randomization, and at fixed time intervals during follow-up.
- The mean eGFR at screening, was 70 ml/min/1.73 m2 and 33% of the patients (n=2,745) had CKD. The median UACR was 1.0 mg/mmol and 24% (n=441) had an increased UACR (micro/macroalbuminuria).
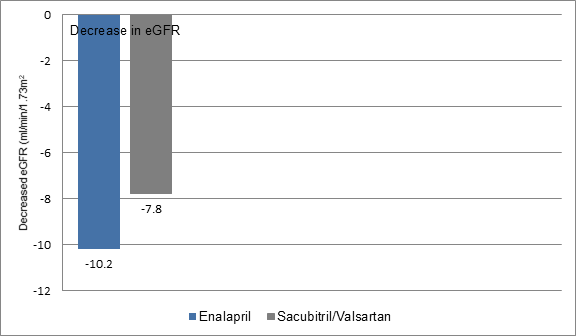
- Patients receiving sacubitril/valsartan had a smaller decrease in the eGFR during follow-up as compared to those on enalapril (7.8 vs. 10.2 ml/min/1.73 m2) (Figure 1)
Figure 1: Decrease in eGFR in the study groups
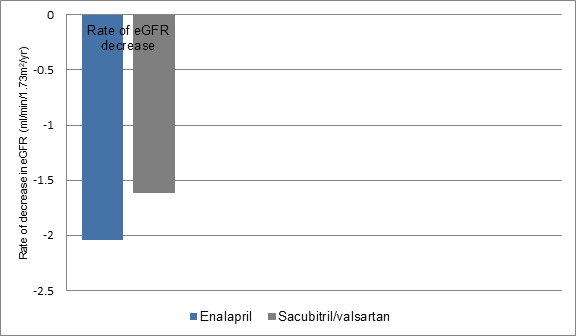
- The rate of eGFR decrease was also lesser in sacubitril/valsartan treated patients vs. those treated with enalapril irrespective of CKD status at screening (-1.61 vs. -2.04 ml/min/1.73 m2/year; p<0.001) (figure 2), despite a greater increase in UACR with sacubitril/valsartan vs. enalapril (1.20 mg/mmol vs. 0.90 mg/mmol; p < 0.001).
Figure 2: Rate of eGFR decrease in study groups
- The worsening of UACR with sacubitril/valsartan was mainly driven by a shift from normoalbuminuria to microalbuminuria at 1 month of follow-up, though this difference remained insignificant at 8 months post-randomization.
- Greater proportion of patients treated with sacubitril/valsartan rather than enalapril experienced an increase of ≥25% in the UACR at 1 month (46% vs. 39%; p=0.004) and 8 months (51% vs. 39%; p < 0.001).
- The incidence of pre-defined renal outcomes did not differ significantly in the study groups, and also amongst those with or without CKD at screening.
- The incidence of conventional renal composite outcomes was 37% lower in the patients treated with sacubitril/valsartan vs. enalapril [hazard ratio (HR); 0.63, p=0.028), irrespective of the CKD status at screening (p for interaction, 0.97).
- Worsening of UACR category was associated with a higher risk of the pre-specified composite renal endpoint in the enalapril group (HR: 4.21; 95% CI: 1.66 to 10.68), but not in the sacubitril/valsartan arm (HR: 0.50; 95% CI: 0.07 to 3.77; p = 0.06 for interaction). Similarly, patients in the enalapril group with a 25% increase in the UACR had a higher risk of prespecified composite renal endpoint (HR; 2.53, 95% CI; 1.09-5.84) vs. those in the sacubitril/valsartan group (HR; 0.28, 95% CI; 0.08-1.01, p for interaction=0.05).
- The impact of sacubitril/valsartan on cardiovascular death or heart failure hospitalization was not altered by eGFR, UACR (p interaction = 0.70 and 0.34, respectively), or by change in UACR (p interaction = 0.38).
- The benefits of sacubitril/valsartan over enalapril were irrespective of increase/decrease in UACR at one month post-randomization vs. the pre-run-in period.
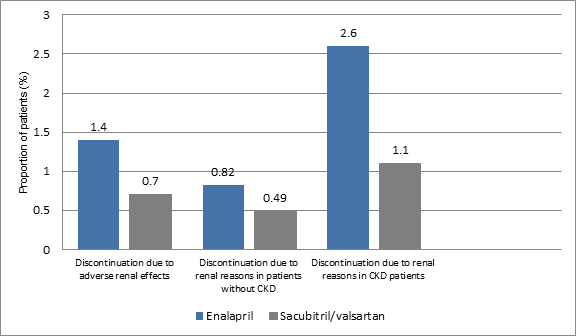
- The discontinuation of medication due to adverse renal effect was slightly higher in enalapril group vs. sacubitril/valsartan group (1.4% vs. 0.7%; p=0.002). The rate of discontinuation amongst the CKD patients was similar in both the study groups (25% vs. 24%, p=0.72) (Figure 3).
- The discontinuation of treatment in patients without CKD due to renal reasons was higher in the enalapril group rather than sacubitril/valsartan group (0.82% vs. 0.49%; HR, 0.59, p=0.12). Similarly, the proportion of patients with CKD who discontinued medication due to renal reasons was higher in enalapril group vs. sacubitril/valsartan group (2.6% vs. 1.1%, HR; 0.43, p=0.008) (Figure 3).
Figure 3: Discontinuation of treatment in the study groups due to renal reasons
Conclusion
- Sacubitril/valsartan resulted in a slower rate of decrease in the eGFR and improved cardiovascular outcomes vs. enalapril, even in patients with CKD, though there was a modest increase in UACR.
JACC Heart Fail. 2018; 6 (6): 489-98.