Introduction
Individuals with glucose-6-phosphate dehydrogenase (G6PD) deficiency are more sensitive to developing haemolytic anaemia after exposure to haemolytic stressors, including oral dapsone.
Aim
To evaluate the risk of haemolysis in subjects with G6PD deficiency treated with dapsone gel 5% or vehicle for acne.
Patient Profile
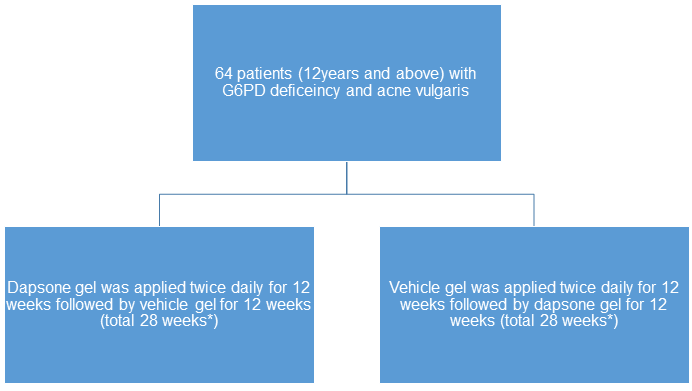
- N=64
- 12 years or older with G6PD deficiency and acne vulgaris
|
Characteristic |
Value a |
|
Age, mean (SD) (range), y |
28 (10) (12-61) |
|
Women |
35 (55) |
|
Ethnic group African American Asian Hispanic Otherb |
56 (88) 4 (6) 1 (2) 3 (5) |
|
G6PD enzyme activity, mean (SD), U/g Hb Severely deficientc Deficientd |
3.8 (1.9) (0.7-6.9) 15 (23) 49 (77) |
|
Lesion count, mean (SD) (range) No.Inflammatory Noninflammatory Totale |
16.2 (12.5) (0-50) 26.5 (25.4) (0-139) 42.8 (27.8) (10-162) |
Abbreviations: G6PD, glucose-6-phosphate dehydrogenase; Hb, hemoglobin.
a Unless otherwise indicated data are reported as number (percentage) of subjects.
b The subjects who identified their ethnic group as other were Middle Eastern, mixed race (white black), and Haitian.
c Severely deficient is defined as 2 U/g of Hb or lower.
d Deficient is defined as higher than 2 U/g Hb up to the lower limit of normal at 7 U/g Hb.
eTotal lesion count is the sum of inflammatory and noninflammatory lesion counts.
Methods
Study Design
- Double-blind, randomized, vehicle-controlled, crossover study
- Blood tests were carried to measure the plasma dapsone and N-acetyldapsone concentrations to evaluate chemistry and haematology parameters
Study Outcomes
Results of clinical chemical analysis and hematology values; plasma dapsone and N-acetyl dapsone concentrations; spontaneous reports of adverse events.
Results
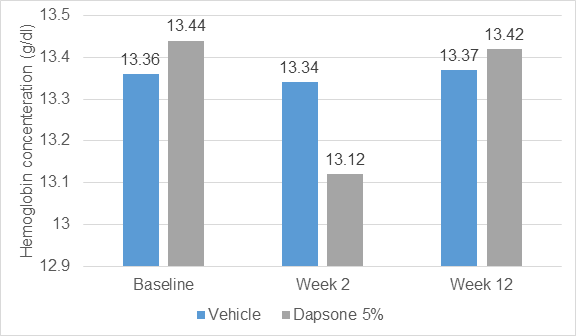
- No change in haemoglobin level at the end of 12 weeks
- A 0.32-g/dL decrease in haemoglobin concentration occurred from baseline to 2 weeks during dapsone gel treatment was not correlated with plasma dapsone levels, amount of daily dapsone gel use (mean, 1g/d), or changes in bilirubin concentration, relative reticulocyte count, haptoglobin concentration, or lactate dehydrogenase levels
- The largest drops in Hb concentration was observed in a safety-evaluable subject occurred during vehicle treatment (a 1.7-g/d decrease at week 2).
- Two of 56 subjects experienced a change in Hb concentration of 1 g/dL or higher with a concomitant increase of bilirubin level to above the upper limit of normal (1 subject at 2 weeks and the other at 12 weeks of dapsone gel treatment) but without any other laboratory changes or clinical signs of haemolytic anaemia (4%)
- No mean change in other laboratory markers of haemolysis at either week 2 or week 12
- Haemolysis parameters were similar in various subgroups, including G6PD enzyme activity, race, sex, and age
- No clinical diagnosis or symptoms of haemolytic anaemia were noted
- No difference in risk of haemolysis in subgroup of severely G6PD deficient (>2 U/g Hb) patients
- No adverse events were reported that were clinical signs or symptoms of haemolytic anaemia.
- 27 of 63 subjects experienced an adverse event regardless of relationship to treatment.
- Three subjects had related adverse events detected by laboratory test during treatment, but none of these were indicative of haemolytic anaemia
Conclusion
- After treatment with dapsone gel 5%, no clinical or laboratory evidence of drug-induced haemolytic anaemia was noted in G6PD-deficient subjects with acne vulgaris
- G6PD deficiency represents a highly sensitive marker for the haemolytic potential of drugs and these finding can be extrapolated to patients with acne and normal G6PD enzyme activity
- Safety profile for topical dapsone gel treatment is excellent, and that the risk of haemolytic anaemia during treatment with dapsone gel for acne vulgaris is remote for all patients, including those with G6PD deficiency
Arch Dermatol. 2008 Dec; 144(12): 1564-70