Introduction
Urinary tract infection (UTI) is one of the most common bacterial infections in adults, affecting considerably more women than men. Antibiotic prescriptions for UTI account for 10-20% of all antibiotic prescriptions in ambulatory care and are second only to antibiotic prescriptions for respiratory tract infections.
Aim
To study whether symptomatic treatment with non-steroidal anti-inflammatory drugs (NSAIDs) is non-inferior to antibiotics in the treatment of uncomplicated lower UTI in women.
Patient Profile
- Women with uncomplicated lower urinary tract infection (UTI)
- Aged 18 to 70 years
Methods
- A randomised, double-blind trial
Study Outcome
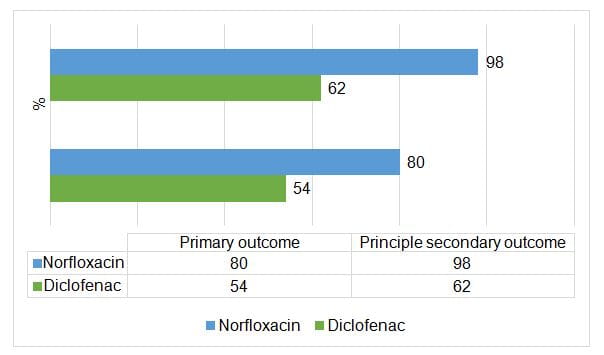
- The primary outcome was symptom resolution on day 3 (72 hours after randomisation and 12 hours after intake of the last study drug)
- The prespecified principal secondary outcome was the use of any antibiotic (including norfloxacin and fosfomycin as trial drugs) up to day 30
Results
- Non-steroidal anti-inflammatory drug (NSAID) diclofenac was inferior to antibiotic treatment with norfloxacin in controlling symptoms
|
Outcomes |
Diclofenac group (n=133) |
Norfloxacin group (n=120) |
Risk or mean difference |
P value |
|
Resolution of symptoms: |
|
|
|
|
|
Day 3 (primary outcome) |
72 (54) |
96 (80) |
27 |
<0.001 |
|
Day 7 |
111 (83) |
115 (96) |
12 |
0.003 |
|
Day 10* |
126 (95) |
116 (97) |
2 |
0.45 |
|
Day 30* |
127 (95) |
111 (93) |
−3 |
0.32 |
|
Complete absence of symptoms: |
|
|
|
|
|
Day 3 |
10 (8) |
20 (17) |
9 |
0.038 |
|
Day 7 |
44 (33) |
65 (54) |
21 |
0.001 |
|
Day 10* |
70 (53) |
77 (64) |
12 |
0.07 |
|
Day 30* |
101 (76) |
99 (83) |
6 |
0.22 |
|
Mean (SD) change of symptom score: |
|
|
|
|
|
Day 3 |
−7.3 (4.7) |
−10.3 (4.1) |
3.0 |
<0.001 |
|
Day 7 |
−11.0 (4.8) |
−12.6 (4.2) |
1.6 |
0.005 |
|
Day 10* |
−12.2 (4.3) |
−12.9 (4.1) |
0.7 |
0.20 |
|
Day 30 |
−13.0 (4.4) |
−13.1 (4.3) |
0.1 |
0.88 |
|
Use of any antibiotic: |
|
|
|
|
|
≤day 3* |
58 (44) |
116 (97) |
−54 |
<0.001 |
|
< day 30 (principal secondary outcome) |
82 (62) |
118 (98) |
−37 |
<0.001 |
|
Use of rescue antibiotic: |
|
|
|
|
|
≤day 3 |
55 (41) |
9 (8) |
34 |
<0.001 |
|
|
73 (55) |
18 (15) |
40 |
<0.001 |
|
Negative urinary culture at day 10 |
96 (72) |
112 (93) |
21 |
<0.001 |
|
Reconsultations because of UTI ( |
27 (20) |
10 (8) |
12 |
0.010 |
|
Mean (SD) quality of life (range 0-10): |
|
|
|
|
|
EuroQol health state (day 3) |
8.8 (2.2) |
9.4 (1.5) |
0.6 |
0.005 |
|
EuroQol visual analogue scale (day 3) |
7.4 (1.9) |
8.3 (1.5) |
1.0 |
<0.001 |
|
Patient satisfaction with UTI management |
5.7 (3.0) |
8.2 (2.1) |
2.5 |
<0.001 |
|
No of working days lost due to UTI |
0.6 (0.8) |
0.5 (0.8) |
0.2 (−0.1 to 0.5)† |
0.18 |
UTI=urinary tract infection; EuroQol=European quality of life instrument.
Positive differences favour norfloxacin, negative differences favour diclofenac.
*Analysis of additional time points, not prespecified in the protocol.
†Relative rate increase calculated from Poisson regression.
- Six women in the diclofenac group (5%) but none in the norfloxacin group received a clinical diagnosis of pyelonephritis (P=0.03)
|
Adverse events |
No (%) in diclofenac group (n=133) |
No (%) in norfloxacin group (n=120) |
Risk difference |
P value* | |
|
Related to UTI |
26 (20) |
10 (8) |
11 |
0.012 | |
|
|
Persistent symptoms |
16 (12) |
4 (3) |
9 |
0.011 |
|
|
Additional symptoms |
6 (5) |
2 (2) |
3 |
0.29 |
|
|
Recurrent UTI† |
5 (4) |
4 (3) |
0 |
1.00 |
|
|
Pyelonephritis‡ |
6 (5) |
0 (0) |
5 |
0.031 |
|
Other adverse event |
17 (13) |
12 (10) |
3 |
0.56 | |
|
|
Exanthema |
1 (1) |
2 (2) |
−1 |
0.61 |
|
|
Vaginitis |
3 (2) |
0 (0) |
2 |
0.25 |
|
|
Gastrointestinal symptoms§ |
3 (2) |
3 (3) |
−0 |
1.00 |
|
Low back pain¶ |
5 (4) |
2 (2) |
2 |
0.45 | |
|
Viral infection |
1 (1) |
3 (3) |
−2 |
0.35 | |
|
Trauma |
3 (2) |
1 (1) |
1 |
0.62 | |
|
Miscellaneous** |
3 (2) |
1 (1) |
1 |
0.62 | |
UTI=urinary tract infection.
Numbers do not add up as patients experienced at least one adverse event for each category.
*Two-sided Fisher’s exact test.
†Recurrent UTI was defined as additional visits after day 14 because of recurrent UTI symptoms after symptoms had resolved by day 10, and the physician decided to treat with antibiotics.
‡One patient in the diclofenac group was admitted to hospital on day 4 because of pyelonephritis.
§Includes one case of diverticulitis in the norfloxacin group.
¶Considered to be of musculoskeletal origin by treating doctor
**Includes one case of external otitis and two cases of tonsillitis in the diclofenac group and one case of hair loss in the norfloxacin group
Conclusion
- The study demonstrated that diclofenac is inferior to norfloxacin for symptom relief of UTI and is likely to be associated with an increased risk of pyelonephritis, even though it reduces antibiotic use in women with uncomplicated lower UTI
- The findings suggested that symptomatic treatment is inferior to antibiotic treatment for women with uncomplicated lower UTI in an ambulatory setting, as it increases median symptom duration by two days and is likely to be associated with an increased risk of clinically diagnosed pyelonephritis
Reference
BMJ 2017;359: j4784