Introduction
LNH-98.5 trial was the first randomized study comparing the standard CHOP chemotherapy regimen to CHOP plus rituximab (R-CHOP) performed by Groupe d’Etudes des Lymphomes de l’Adulte (GELA). The 2-year results demonstrated that the addition of rituximab to the CHOP regimen resulted in favorable outcomes in elderly patients with diffuse large B-cell lymphoma (DLBCL), with a greater proportion of complete responders and longer event-free and overall survival compared with CHOP alone.
Aim
To compare long term outcomes of the standard CHOP chemotherapy regimen to CHOP plus rituximab (R-CHOP) in elderly patients with DLBCL
Patient Profile
- Patients with DLBCL aged 60 to 80 years
- Previously untreated DLBCL according to the World Health Organization classification, stage II, III, or IV disease, and performance status (PS) 0 to 2 according to the Eastern Clinical Oncology Group (ECOG) scale
Methods
- Patients were randomized to treatment with either standard CHOP therapy or CHOP plus rituximab
Study Drugs
- CHOP: Patients treated with CHOP received 750 mg/m2 cyclophosphamide, 50 mg/m2 doxorubicin, 1.4 mg/m2 vincristine up to a maximum dose of 2 mg on day 1, and 40 mg/m2/day prednisone on days 1 to 5, for each treatment cycle
- R-CHOP: Patients treated with R-CHOP received rituximab at a dose of 375 mg/m2 on day 1 of each of the 8 cycles along with CHOP regimen
Median Follow-up Duration: 10 years
Study Endpoints
- The primary efficacy parameter was event-free survival (EFS). Events were defined as the following: disease progression or relapse, initiation of a new (unplanned) anticancer treatment (e.g., radiation therapy), or death from any cause without progression
- Secondary efficacy end points included overall survival (OS), progression-free survival (PFS), disease-free survival (DFS), response rates, and toxicity
Results
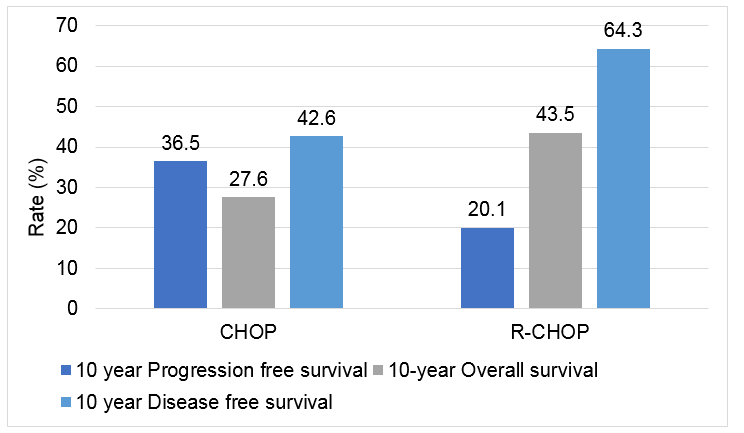
- The addition of rituximab improved PFS and OS rates in this patient population, with an overall increase of 16% versus CHOP alone.
|
Type of event |
CHOP |
R-CHOP |
|
PD during treatment |
44 (22.3%) |
19 (9.4%) |
|
New unplanned treatment |
9 (4.6%) |
11 (5.4%) |
|
Progression after stable disease |
1 (0.5%) |
1 (0.5%) |
|
PD after partial response |
5(2.5%) |
6 (3.0%) |
|
Relapse for CR patients |
71 (36.0%) |
49 (24.3%) |
|
Death without PD during treatment |
12 (6.1%) |
12 (5.9%) |
|
Death without PD after treatment |
16 (8.1%) |
33 (16.3%) |
|
No event |
39 (19.8%) |
71 (35.1%) |
CHOP: cyclophosphamide, doxorubicin, vincristine, and prednisone; R-CHOP: rituximab-CHOP; PD: progressive disease; and CR: complete response
- The median OS after progression was 0.6 and 0.7 months for the CHOP and R-CHOP arms, respectively
- Median DFS was 3.4 years in the CHOP arm and was not yet reached in the R-CHOP arm (P <.0001)
- Some patients responded well to salvage therapy and had a relatively long survival period after progression
- The 5- and 10-year survival after progression were 14.6% and 10.5% in the CHOP arm, compared with 25.0% and 8.6% in the R-CHOP arm, respectively
- Overall, the number of patients exhibiting late disease progression was low, but there was a trend toward a better outcome for those patients treated with R-CHOP compared with those treated with CHOP alone
|
PD |
Median survival (y) |
2-year survival (%) |
3-year survival (%) |
5-year survival (%) |
|
PD within the first 3 years |
|
|
|
|
|
CHOP |
0.6 |
25.9 |
19.6 |
14.3 |
|
R-CHOP |
0.6 |
18.2 |
18.2 |
16.7 |
|
PD between years 4 and 5 |
|
|
|
|
|
CHOP |
3.0 |
83.3 |
50.0 |
16.7 |
|
R-CHOP |
not reached |
83.3 |
66.7 |
66.7 |
|
PD after 5 years |
|
|
|
|
|
CHOP |
0.9 |
22.2 |
22.2 |
22.2 |
|
R-CHOP |
not reached |
87.5 |
87.5 |
58.3 |
PD: progressive disease; CHOP: cyclophosphamide, doxorubicin, vincristine, and prednisone; and R-CHOP: rituximab-CHOP.
- The same risk of death due to other diseases, secondary cancers, and late relapses was observed in both study arms
- Relapses occurring after 5 years represented 7% of all disease progressions
Conclusion
- The addition of rituximab improved PFS and OS rates in this patient population, with an overall increase of 16% versus CHOP alone
- The patient cohort chosen for this trial was elderly DLBCL patients (60-80 years of age), a particularly challenging group to manage and treat
- With the improvements in PFS and OS rates from the addition of rituximab to standard treatment regimens, a significant proportion of elderly patients experience long-term survival
- The 10-year analysis confirmed the benefits and tolerability of the addition of rituximab to CHOP
- Late relapses can be expected in DLBCL patients if the follow-up period is sufficiently long
- The risk of death due to other diseases or secondary cancers was not higher in the R-CHOP group
- These findings confirm that the use of R-CHOP can improve patient outcomes in elderly DLBCL patients, and that the beneficial effects are sustained over a long follow-up period
Reference
Blood. 2010; 116(12):2040-2045