Tinidazole versus Metronidazole in Treatment of Amoebic Liver Abscess
Introduction
Metronidazole and tinidazole are both effective amoebicidal drugs for intestinal and hepatic amebiasis, of which metronidazole has long been preferred by the clinicians; but metronidazole has been associated with several side effects and non-response.
Aim
To evaluate the efficacy and safety profile of tinidazole as compared to metronidazole in the treatment of amebic liver abscess (ALA).
Patient Profile
Consecutive patients with ALA admitted to the Department of Gastroenterology, Sawai Man Singh (SMS) Medical College and Hospital, Jaipur, India
|
Parameters |
Group M (n = 75) |
Group T (n =75) |
p-value |
|
Age (± SD) |
38.3 (± 13.29) |
39.4 (± 13.3) |
0.61 |
|
Gender |
|
|
|
|
F (%) |
9 (12) |
9 (12) |
0.80 |
|
M (%) |
66 (88) |
66 (88) |
|
|
Socioeconomic status |
|
|
|
|
Low (%) |
58 (77.3) |
53 (70.7) |
0.16 |
|
Middle (%) |
13 (17.3) |
12 (16) |
|
|
Upper (%) |
4 (5.3) |
10 (13.3) |
|
|
Personal |
|
|
|
|
Alcohol (%) |
39 (52) |
21 (28) |
0.07 |
|
Smoker (%) |
30 (40) |
21 (28) |
0.16 |
|
Clinical features |
|
|
|
|
Pain (%) |
69 (92) |
70 (93.3) |
1.0 |
|
Fever (%) |
65 (86.6) |
63 (82) |
0.12 |
|
Jaundice (%) |
15 (20) |
14 (18.6) |
1.0 |
|
Chest pain (%) |
34 (45.3) |
24 (32) |
0.13 |
|
Pedal edema (%) |
29 (38.6) |
22 (29.3) |
0.30 |
|
Dysentery (%) |
8(10.6) |
7 (9.3) |
1.0 |
|
Tender hepatomegaly (%) |
65 (86.6) |
67 (89.3) |
0.22 |
|
Intercostal tenderness (%) |
61 (81.3) |
53 (70.6) |
0.18 |
|
Laboratory parameters |
|
|
|
|
Hemoglobin (g/dL, ± SD) |
11.06 (± 1.75) |
11.55 (± 1.74) |
0.08 |
|
TLC/mm3 (± SD) |
2.0 × 104 (± 4.0 × 104) |
1.5 × 104 (± 1.0 × 104) |
0.37 |
|
Platelet count/mm3 (± SD) |
3.06 × 105 (± 1.5 × 105) |
2.96 × 105 (± 1.3 × 105) |
0.67 |
|
Bilirubin (mg/dL, ± SD) |
2.01 (± 1.6) |
1.58 (± 1.4) |
0.10 |
|
SAP IU/L (± SD) |
226 (± 98.7) |
201.81 (± 71.57) |
0.08 |
|
S Cr mg/dL |
1.05 (± 0.5) |
1.08 (± 0.5) |
0.79 |
|
Albumin (g/dL, ± SD) |
2.87 (± 0.5) |
2.96 (± 0.5) |
0.33 |
|
X-ray chest |
|
|
|
|
Pleural effusion (%) |
15 (20) |
19 (25.3) |
0.55 |
|
Elevated diaphragm (%) |
19 (25.3) |
17 (22.6) |
0.61 |
|
Location on USG |
|
|
|
|
Right lobe (%) |
53 (70.6) |
60 (80) |
0.25 |
|
Left lobe (%) |
10 (13.3) |
8 (10.6) |
0.8 |
|
Both lobes (%) |
12 (16) |
7 (9.3) |
0.32 |
|
USG-guided therapeutic aspiration |
|
|
|
|
|
34 (45.3) |
24 (31.9) |
0.13 |
F female, M male, TLC total leukocyte count, SAP serum alkaline phosphatase, S Cr serum creatinine, USG ultrasonography
Methods
- Randomized controlled trial with two parallel treatment groups
Treatment
- All the patients were started on intravenous (I/V) antibiotics initially and shifted to oral treatment once fever and pain were relieved
- Patients were started on either metronidazole, I/V 2–2.5 g/day then oral 2–2.4 g/day for 14 days, or tinidazole I/V 1.6 g/day then oral (2 g/day) for 5 days according to group assigned
Study outcomes
- Follow up was done at 1, 3, and 6 months
- Patients were observed for fever and abdominal pain, laboratory parameters, and side effects of study drugs
- The early clinical response was defined as the absence of fever and abdominal pain within 72 h of starting treatment
- The symptomatic clinical response (SCR) was defined as the absence of fever and abdominal pain irrespective of the duration of treatment required
- Patients were followed up clinically, biochemically, and radiologically (abdominal USG) at 1, 3, and 6 months
Results
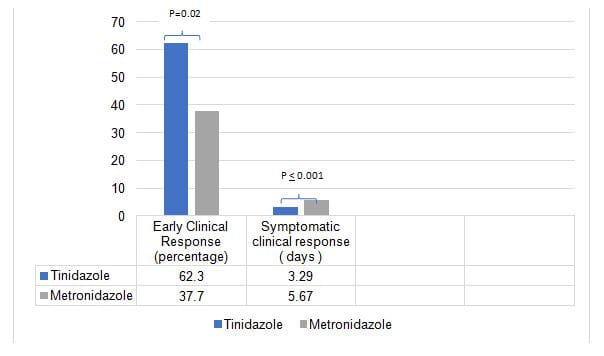
- Percentage of patients showing early clinical response was higher in the tinidazole than in the metronidazole group
- Patients receiving tinidazole showed symptomatic clinical response which was significantly lower compared to patients receiving metronidazole
- SCR was shorter in group T than group M
- Mean residual volume at the end of 1 month was lower in group T and no significant difference was seen at 3 and 6 months
|
|
Group M (mean ± SD) |
Group T (mean ± SD) |
p-value |
|
Abscess volume baseline (mL ± SD) |
336.56 (± 264.8) |
291.11 (± 231.9) |
0.26 |
|
1 month |
184.72 (± 143.3) |
130.76 (± 108.1) |
0.01 |
|
3 month |
96.05 (± 79.4) |
78.52 (± 89.0) |
0.25 |
|
6 month |
38.42 (± 44.8) |
33.60 (± 65.5) |
0.64 |
|
Difference (1 vs. 0 month) |
− 131.74 (± 114.1) |
− 145.06 (± 122.8) |
0.51 |
|
Difference (3 vs. 0 month) |
− 209.68 (± 170.9) |
− 189.46 (± 146.5) |
0.48 |
|
Difference (6 vs. 0 month) |
− 271.12 (± 208.2) |
− 232.15 (± 180.3) |
.27 |
|
|
Group M Number (%) |
Group T Number (%) |
p-value |
|
1 month |
0 |
2 (2.6%) |
0.77 |
|
3 months |
15 (20%) |
19 (25.3%) |
0.55 |
|
6 months |
32 (42.6%) |
37 (49.3%) |
0.52 |
- Tinidazole was better tolerated with fewer side effects
- Low socioeconomic status, baseline abscess volume > 500 mL, hypoalbuminemia, pleural effusion, and history of ethanol use were associated with a late clinical response on univariate analysis of which low socioeconomic status was the only associated factor
Conclusion
- The study demonstrated that the use of tinidazole as compared to metronidazole is associated with
- early clinical response
- shorter course of treatment
- the favourable rate of recovery
- high tolerability
- Tinidazole should be preferred over metronidazole in treatment of amoebic liver abscess
Reference
Indian J Gastroenterol.2018 May;37(3):196-201