Treating Functional Constipation in Children: PEG 3350 vs. Lactulose
10 Mar, 21
Introduction
Polyethylene glycol (PEG) is the recommended first-line laxative for both fecal disimpaction and maintenance treatment in pediatric patients suffering with functional constipation (FC). Lactulose may be used in patients in case of PEG intolerance or unavailability.
Aim
To compare the clinical efficacy and tolerance of PEG 3350 and lactulose for the treatment of FC in infants and children
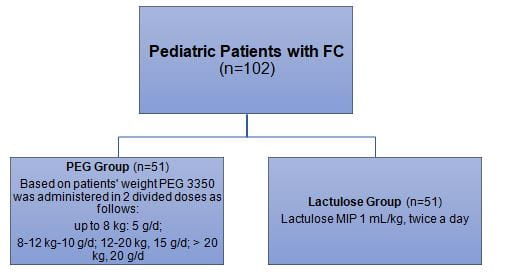
Patient Profile
- Pediatric patients with diagnosis of FC as per the Rome III criteria, and children with Fc who were previous ineffective treatment (age; 6 months-6 years)
Methods
Study Design
- A randomized, multicenter study
Treatment Strategy
- Patients were randomized to receive either PEG 3350 or lactulose.
Treatment Duration
- 12 weeks
Follow-up Visits
- At week 4
- Telephonic follow-up at week 16
Outcomes
Primary Outcomes
- The number of defecations per week after 12 weeks of treatment
- Improvement in stool consistency of at least 2 points on the Bristol scale
Secondary Outcomes
- The incidence of adverse events
- Bowel movements ≥ 3 per week
- Stool consistency ≥ 2 (Bristol scale) as successful treatment
Results
- Mean age of the study population was 3.62 years, 88 patients completed the
- study.
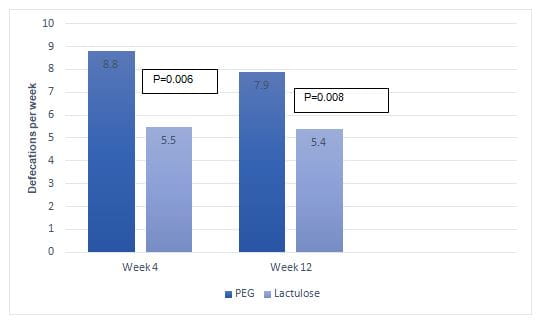
- At week 4, good clinical outcomes were evident in 100% vs. 80% patients in PEG and lactulose group, respectively (p=0.001). At week 4, patients in the PEG group had significantly more defecations per week when compared with patients in the lactulose group (8.5 vs. 5.5; p=0.006). Patients in the PEG group rather than lactulose group also experienced superior improvements in additional symptoms such as defecation with pain, stool retention, large volume of stools, and hard stools; though the between group difference was insignificant.
- Five patients (10%) in the lactulose group shifted to PEG group at week 4 due to lack of effect of lactulose or intolerance
- At week 12, patients in PEG as well as lactulose groups achieved good clinical outcome (98% vs. 90%; p=0.13). At 12 weeks, patients in the PEG group had significantly more defecations per week as compared with those in the lactulose group (Fig 1). The improvement in additional symptoms including; frequency of defecation with pain (5% vs. 5%; p=0.9), stool retention (7% vs. 10%; p=057), large volume of stools (30% vs. 31%; p=0.9) and hard stools (7% vs. 13%; p=0.58) did not differ significantly between the two study groups.
Fig 1: Improvement in bowel movement in the study groups
- The mean stool consistency at week 12 on the Bristol scale was 4.
- At week 16, 5 patients from the lactulose group had shifted to PEG group. Good clinical outcomes were observed in 89% vs. 82% patients in the PEG and lactulose groups, respectively
- Patients in the lactulose group experienced significantly greater adverse events (mostly bloating, gases or abdominal pain) at weeks 4 (27 vs. 38; p=0.04) and 12 (15 vs. 23; p=0.02), when compared with those in the PEG group.
Conclusions
- Amongst children with FC; including those aged less than two years, PEG 3350 was more effective and resulted in fewer adverse events than lactulose.
J Pediatr Gastroenterol Nutr. 2019;68(3):318-324.
Related Topics