To compare systemic pharmacodynamic effects of the test product, formoterol fumarate HFA pMDI (FM-Test, Cipla Ltd, India) with the reference product Foradil(R) HFA pMDI (FM- Reference supplied by Novartis, Italy) in healthy adult male human subjects
Comparison of Systemic Pharmacodynamic Effects of Formoterol (Cipla) with Foradil (Novartis)
18 Mar, 14
Comparison of Systemic Pharmacodynamic Effects of Two HFA Formulations of Formoterol Fumarate
Objective
Methods
- Randomised, placebo controlled, double-blind study
- Healthy subjects were assigned to one of the two groups to receive the study drug in a crossover manner on five separate days
- Each treatment day was separated by a 3 day washout period
- Group I - Test or reference 12 mcg (1 puff) OR Test or reference 48 mcg (4 puffs) OR Placebo
- Group II - Test or reference 24 mcg (2 puffs) OR Test or Reference 96 mcg (8 puffs) OR Placebo.
- Heart rate, QTc interval, blood pressure and venous sampling for serum potassium and plasma glucose were measured over 12 hrs post dosing
- Primary endpoints were maximum heart rate (0-12 hours) during 12 hr after dosing and minimum serum potassium (0-4 hrs) during 4 hr after dosing
Results
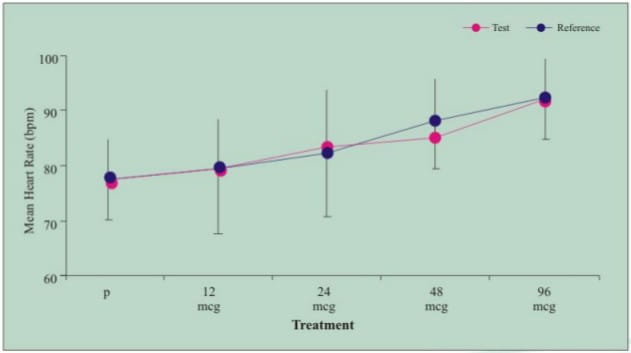
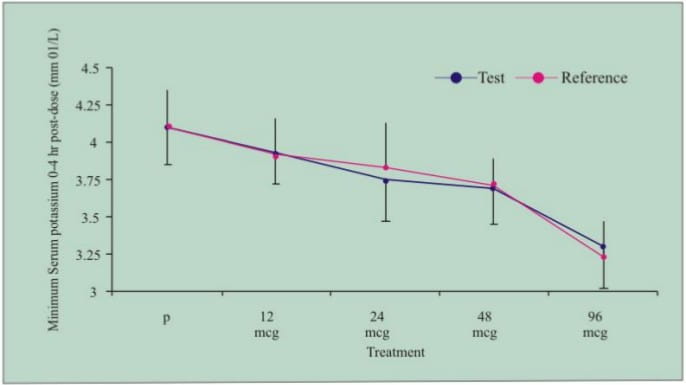
- Both the test and reference product demonstrated dose-related beta agonist pharmacodynamic effects in heart rate (Figure 1) and serum potassium (Figure 2) as compared with placebo (P)
Figure 1: Mean (SD) maximum heart rate (0-12h) post dose following administration of formoterol 12 mcg, 24 mcg, 48 mcg, 96 mcg and placebo (P) in healthy volunteers
Figure 2: Mean (SD) minimum serum potassium (0-4h post dose) following administration of formoterol 12 mcg, 24 mcg, 48 mcg, 96 mcg and placebo (P) in healthy volunteers
- The 95% CI for maximum heart rate (0-12 hr post dose) and minimum serum potassium (0-4 hr post dose), was within the equivalence limits of ± 10 bpm for maximum heart rate and ± 0.33 mmol/L for minimum serum potassium at the recommended doses of 12 and 24 mcg, for both the test and reference product.
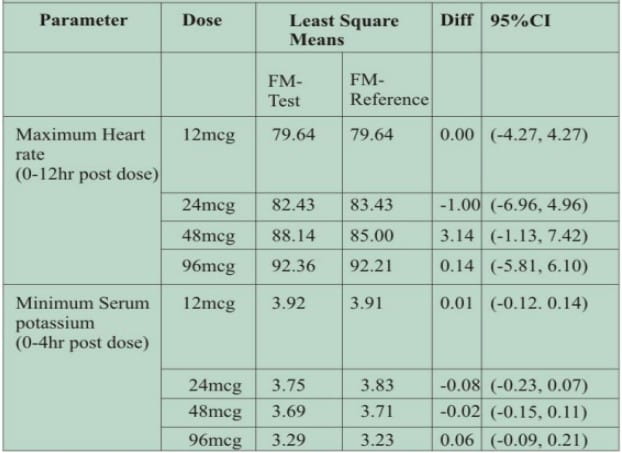
- Even at supratherapeutic doses of 48 mcg and 96 mcg, no significant difference was observed in maximum heart rate and minimum serum potassium (Table 1)
Table 1: Mean maximum heart rate (0-12 hr) and mean minimum serum potassium (0-4 hr) after administration of the two formulations of formoterol.
- There were no differences in the secondary endpoints for maximum QTc interval, maximum systolic blood pressure, minimum diastolic blood pressure and maximum plasma glucose
- Safety - Out of the 24 treatment emergent adverse events, 20 were expected and related to the study medication. The most commonly reported adverse event was hypotension and tremor.
Conclusion
- The test product and reference product showed comparable dose related effects on primary endpoints, heart rate and serum potassium
- At recommended doses of 12mcg and 24 mcg, both formulations showed equivalent effects on pulse rate, maximum heart rate and minimum serum potassium post dose.
-
Thus the test product formoterol fumarate HFA pMDI (Cipla) was found to be equivalent to reference product Foradil HFA pMDI (Novartis) and both treatments were well tolerated at all given doses.
P2042, presented at European Respiratory Society (ERS) Conference, 2009