- This study was conducted in patients with moderate to severe active RA who had inadequate response to DMARDs.
- Eligible patients received Etanercept 25 mg twice weekly through subcutaneous route for 24 weeks.
- Efficacy assessment was carried out at baseline visit (visit-3), 2 weeks, 6 weeks, 12 weeks, 18 weeks and 24 weeks after visit 3.
Efficacy and Safety of ETACEPT in Indian Patients with Severe to Moderate Active Rheumatoid Arthritis
18 Mar, 14
Efficacy & Safety of Etacept in Indian Patients
Aim
To demonstrate the safety and efficacy of ETACEPT (etanercept) 25 mg in patients with moderate to severe active rheumatoid arthritis.
Inclusion criteria
- Male or female patients aged ≥18 to 70 years.
- Definite diagnosis made in line with American College of Rheumatology (ACR) 1987 revised criteria.
- Patients having inadequate response to at least one but not more than four DMARDs e.g. Hydroxychloroquine, Sulfasalazine, oral or injectable gold, D-penicillamine, including methotrexate.
- Patients willing to continue the appropriate dose of Methotrexate and discontinuing other DMARDs for rest of the study duration if the patient was maintained on DMARDs during screening.
- ESR (Westergren) ≥28mm/h
- Stabilizing the dosage of NSAIDs at least for 4 weeks before the enrolment if the patient was found taking oral NSAIDs during screening.
- Stabilizing the dosage of oral glucocorticoids at least for 4 weeks (≤10 mg/day equivalent to prednisone) if the patient was taking oral glucocorticoids during the screening.
Study Design
- An open-label, prospective, non-comparative, multicentre study
- N= 104, Mean Age- 47 years, Mean disease duration- 2.57 years
Treatment Regimen
Assessment Parameters
Primary Efficacy Criterion
ACR20
Secondary Efficacy Criterion
Reduction in the rate and amount of NSAlDs consumed.
Safety Parameters
- Incidence of all adverse events.
- Incidence of drug-related adverse events.
- Results of safety laboratory examination: haematology, biochemistry, urine alysis and Global assessment of tolerability.
- Patients completing at least 12 weeks of treatment were considered for the statistical evaluation of the efficacy parameters.
- Patients receiving at least one dose of the study medication were considered for safety evaluation.
Results
Primary Efficacy Criterion
Number of patients achieving ACR 20 and improvement over time in individual symptom.
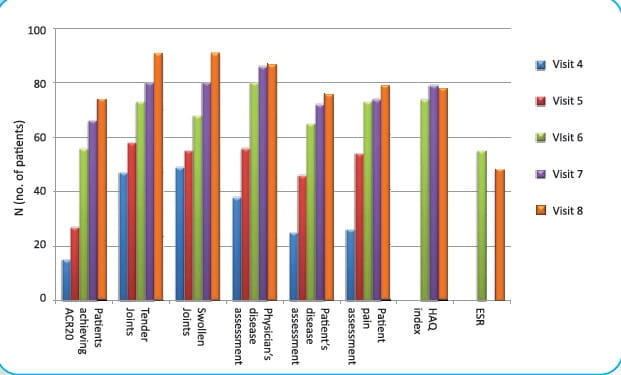
Number of patients (%) achieving ACR 20
- Visit 4- 2 weeks: 15 (15.31%)
- Visit 5- 6 weeks: 27 (27.55 %)
- Visit 6- 12 weeks: 56 (57.14%)
- Visit 7- 18 weeks: 66 (67.35%)
- Visit 8- 24 weeks: 74 (75.51%)
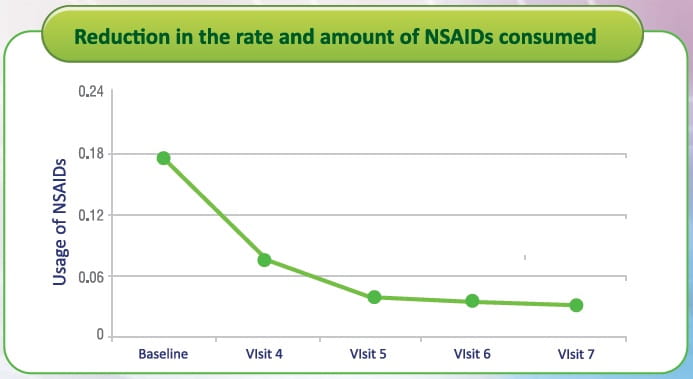
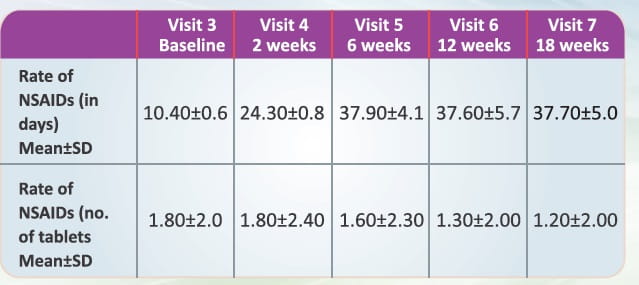
- There was a statistically significant improvement with respect to mean rate of NSAIDs used from baseline to subsequent visits as the p-value at each visit was less than 0.05.
- There was a statistically significant improvement with respect to mean amount of NSAIDs used from baseline to subsequent visits (except at visit 4 and visit 5) as the p-value at each visit (except at visit 4 and visit 5) was less than 0.05.
Safety Analysis
Etacept was well tolerated during the study duration by patients with moderate to severe rheumatoid arthritis.
The common adverse events that were reported during the study duration were as follows:
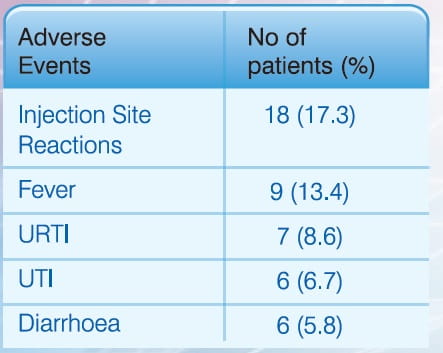
- Most adverse events were not related to the study drug; except for some possibly related events.
- Few injection site reactions were observed; which are expected reactions.
- No patient developed TB.
- No clinically important changes in laboratory parameters or ECG were seen.
- No deaths were reported during the course of this study
Conclusion
In summary, ETACEPT is proven to be highly efficacious and has no significant safety concerns during the administration over a 24 weeks period in the Indian population.
Data on file.










