Fluticasone Inhaler (Cipla) Compared to Flixotide Inhaler
18 Mar, 14
Fluticasone Inhaler (CIPLA) Similar in Efficacy and Safety Compared to Flixotide Inhaler (Gsk)
Objective
- To prove the non-inferiority in terms of efficacy and safety of two fluticasone propionate (FP) inhalers in patients with moderate persistent asthma
Patients and Methods
- Double-blind, double dummy, randomised, multicentre, parallel group, 12 week study
- 308 patients were randomised to receive either fluticasone (Flohale inhaler (FP) CIPLA (n=151) or FLIXOTIDE inhaler (FP) GSK (n=157), 125 mcg/actuation, two puffs bid.
Results
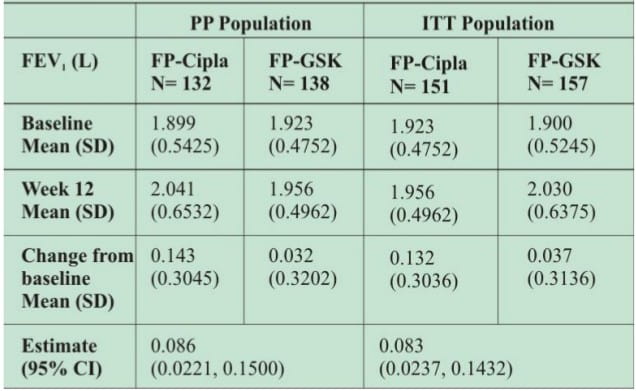
- Non-inferiority of FP-Cipla to FP-GSK was clearly demonstrated for the primary efficacy variable-change from baseline FEV1 for both the (intent-to-treat) ITT and (per-protocol) PP population.
Table 1: Mean change from baseline in FEV1 at week 12
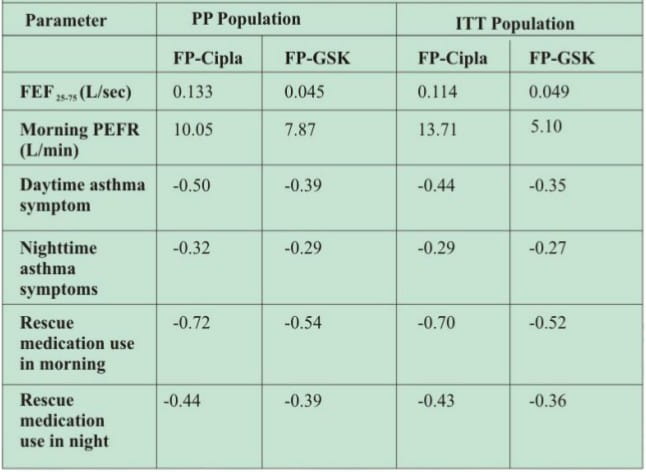
- The secondary efficacy variables were also comparable between the two groups.
Table 2: Mean change from baseline in secondary efficacy parameters
- Both the formulations were well tolerated. The test group reported 74 adverse events compared to 82 in the reference group. Majority of the adverse events were mild to moderate in nature.
Conclusion
- The test fluticasone inhaler (FP-Cipla) was found to be clinically non-inferior to the reference fluticasone inhaler (FP-GSK). Also both the formulations were found to be safe and well-tolerated.