To assess the antihypertensive efficacy and safety of a fixed-dose combination of amlodipine and atenolol (Amlopres-AT) in Indian patients
Postmarketing Surveillance Study on Amlopres-AT (Amlodipine/Atenolol Combination)
19 Apr, 14
Efficacy and tolerability of a fixed-dose combination of amlodipine and atenolol (Amlopres-AT) in Indian hypertensives
A Post Marketing Surveillance Study
Aim
Study Patients
Patients with moderate hypertension or hypertension not controlled by either amlodipine or atenolol alone (N=370)
Study Medication
- Amlopres-AT (Amlodipine 5 mg and atenolol 50 mg) once daily Dosage increased or decreased or maintained at 2 weeks as per patient response
Study Duration
4 weeks
Results
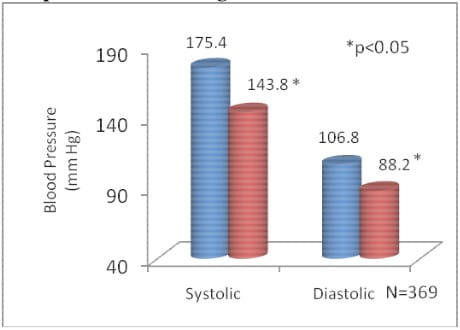
- Treatment with Amlopres-AT showed significant reduction in blood pressure
- Satisfactory BP control was observed in 80.5% of patients with Amlopres-AT
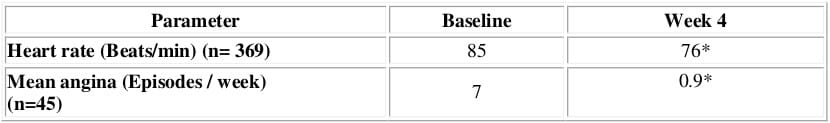
- The combination showed significant reduction in heart rate and also reduced the frequency of angina attacks in patients with concomitant angina
- Treatment with Amlopres-AT was associated with a low incidence of side effects. Commonly seen side effects were oedema, fatigue and headache.
Conclusion
The once daily regimen of the fixed-dose combination of amlodipine and atenolol (Amlopres-AT) provided a significant antihypertensive effect and was well tolerated in Indian patients
The Indian Practitioner 1997: 50; 683-688

.svg?iar=0&updated=20230109065058&hash=B8F025B8AA9A24E727DBB30EAED272C8)