Safety and Efficacy of Beclomethasone in Patients with Asthma
18 Mar, 14
Beclomethasone PMDI is Safe and Efective in Indian Asthmatics
Objective
- To compare safety of Beclomethasone-HFA (Beclate; Cipla Ltd., India) via pressurized metered dose inhaler (pMDI) versus Beclomethasone-CFC pMDI (Allen and Hanburys, UK).
Patients and Methods
- Randomized, open-label, parallel-group study.
- Subjects aged 18-70 years with confirmed diagnosis of asthma.
- 106 patients were included and randomised to 12 week treatment with either Beclomethasone-HFA (400-1200 mcg/day) or Beclomethasone-CFC (400-1200 mcg/day) or a placebo pMDI.
Results
1. Efficacy
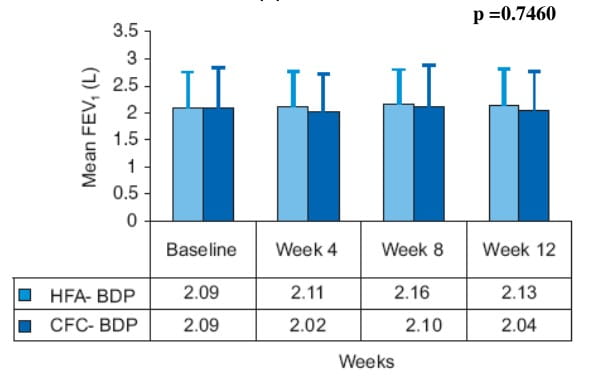
- There were no significant differences between the treatment groups (Beclomethasone-HFA pMDI vs. Beclomethasone-CFC pMDI) in mean values of FEV1 over 12 weeks.
Mean FEV1 (L) over 12 weeks
- There was no significant difference in both the treatment groups for mean daytime and night-time asthma symptom scores at week 12.
- The use of rescue medication was low throughout the study (mean of <1 puff/day).
2. Safety and Tolerability
- There were no significant changes between both the treatment groups at week 12 in the mean change of corrected Urine Cortisol/Creatine (UCC) ratio from baseline (p < 0.9805).
- Only 4 patients out of 106 experienced adverse events. Adverse events reported were oral candidiasis, back pain and upper respiratory tract infection.
- All the adverse events were not related to the study medication and all either resolved or improved with treatment.
Conclusion
- Beclomethasone-HFA pMDI was well tolerated when used in the long-term treatment of adults with mild to moderate persistent asthma.
-
Clinical efficacy of Beclomethasone-HFA pMDI was maintained throughout the study.
3285, presented at European Respiratory Society (ERS) Conference, 2005