Safety and Efficacy of Ciclesonide in Patients with Asthma
18 Mar, 14
Ciclesonide PMDI is Safe and Effective in Indian Asthmatics
Objective
- To determine the efficacy and safety of 200 mcg Ciclesonide (Ciclohale; 160 mcg ex-actuator; Cipla Ltd., India) delivered via HFA pressurized metered dose inhaler (pMDI) in mild to moderate persistent asthma patients.
Patients and Methods
- Open-label, non-comparative, multicenter study.
- 100 patients aged 18 years and above were included and given treatment with 160 mcg Ciclesonide (2 puffs of 80 mcg via HFA pMDI) once daily for 6 weeks.
- Primary efficacy variable was change from baseline in FEV1 and secondary efficacy variables were change from baseline in morning PEFR, total symptom scores and use of rescue medication.
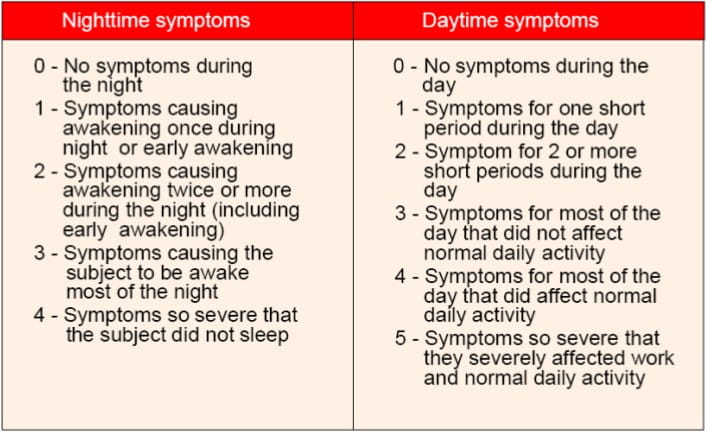
- The total symptom scores were the sum of daytime and nighttime symptoms. Night-time symptom scores were graded on a 5-point scale and daytime symptoms like breathlessness, cough and wheeze were graded on a 6-point scale as follows:
- Other assessments included urinary cortisol levels, biochemical, hematological and ECG at week 6 from baseline.
Results
1. Efficacy
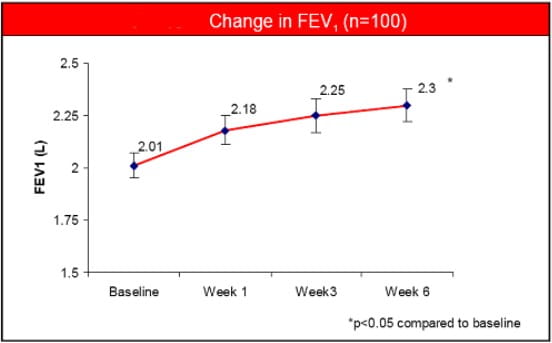
- There was a significant improvement in FEV1 at the end of 6 weeks.
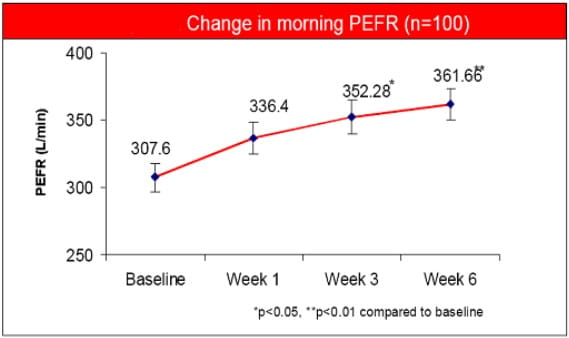
- PEFR showed significant improvement after 3 weeks of treatment.
- There was a significant improvement in the nighttime and daytime symptom scores after 1 week of treatment with inhaled Ciclesonide (p<0.001 compared to baseline).
- There was a significant reduction in the use of rescue medication namely Salbutamol after 1 week of treatment with inhaled Ciclesonide (p<0.001 compared to baseline).
2. Safety
- There was no change in the blood pressure, pulse and respiratory rate, hematological and biochemical parameters throughout the study period.
- Only few patients reported adverse events and most commonly seen were cough, breathlessness, headache, itching in throat/pharyngitis and hoarseness of voice.
- There were no significant differences in the urinary cortisol levels corrected for creatinine (mcg/mg) from baseline to 6 weeks.
Conclusion
-
Ciclesonide 160 mcg HFA pMDI is efficacious and safe for the treatment of mild to moderate asthma.
1242, presented at European Respiratory Society (ERS) Conference, 2006