Safety and Efficacy of Ipratropium + Salbutamol in Patients with COPD
18 Mar, 14
Ipratropium + Salbutamol PMDI is Safe and Efective in Indian Copd Patients
Objective
- To compare efficacy and safety of Ipratropium+Salbutamol-HFA pMDI (Duolin; 20/100 ?g; Cipla Ltd., India) versus Ipratropium+Salbutamol-CFC pMDI (Combivent; 20/100 ?g; Boehringer Ingelheim, UK).
Patients and Methods
- Randomized, double-blind, parallel-group, multicentre study.
- Subjects aged 40-75 years with confirmed diagnosis of COPD according to GOLD guidelines.
- 290 subjects were included and randomised to a 12 week treatment with 2 puffs four times a day of either Ipratropium+Salbutamol-HFA pMDI or Ipratropium+Salbutamol-CFC pMDI or a placebo pMDI.
- The dose of the medication was administered and spirometry was performed before and 60 minutes post-dose.
Results
1. Efficacy
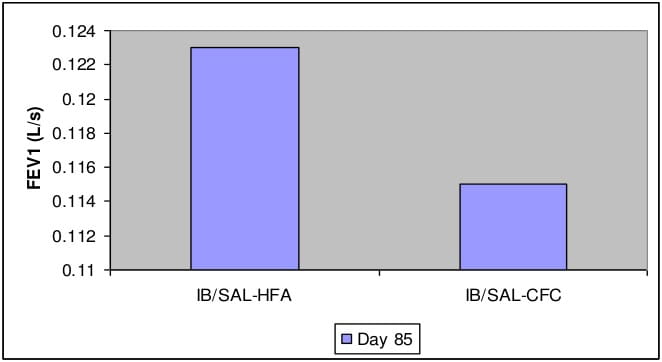
- Non-inferiority of Ipratropium+Salbutamol-HFA pMDI to Ipratropium+Salbutamol-CFC pMDI was clearly demonstrated by change in FEV1 from pre-dose at 60 minutes on day 85.
Change in FEV1 (L/s) from pre-dose at 60 minutes on day 85
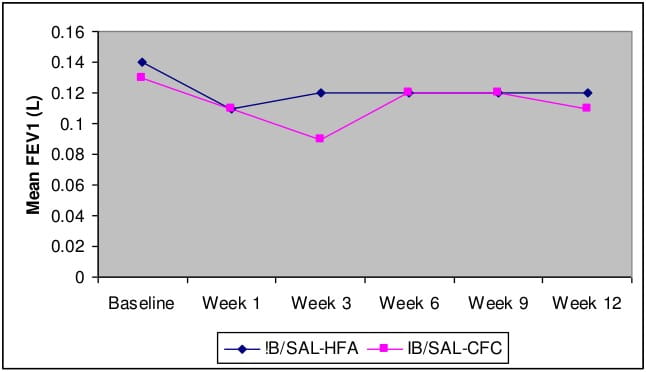
- Mean values of FEV1 over 12 weeks were similar for both the treatment groups (Ipratropium+Salbutamol-HFA pMDI vs. Ipratropium+Salbutamol-CFC pMDI).
Mean FEV1 (L) over 12 weeks
2. Safety and Tolerability
- The incidence of adverse events was similar in the two treatment groups.
- Most common adverse events were headache, productive cough, cough and nasopharyngitis.
Conclusion
- Ipratropium+Salbutamol-HFA pMDI is clinically non-inferior to Ipratropium+Salbutamol-CFC pMDI.
-
The safety profile of the two treatment groups was also similar.
P3593, presented at European Respiratory Society (ERS) Conference, 2007
Related Topics