This was an open-label, prospective, non-comparative, single-centre study. The objective was to evaluate the safety and efficacy of Vitamin C 15% serum for the treatment of photodamaged facial skin.
Vitamin C 15% Serum (VC15) in Patients with Photodamaged Facial Skin
Objective
Methodology
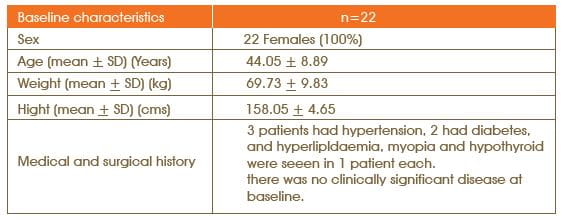
Totally, 22 patients of age 18 years and above were screened for inclusion and exclusion criteria after obtaining a written informed consent The study schedule consisted of four visits.
Patients who satisfied the selection criteria were enrolled into 12 weeks of treatment period. These patients received one bottle of Vitamin C 15% for the treatment of photodamaged facial skin. The patients were advised to apply 7 drops once daily on face at morning. Pre- and post-treatment photographic assessments were made.
Follow-up of the patients was done at weeks 4, 8 and 12 from the baseline visit. At each visit, the investigator noted down the vital signs and a skin examination was done with the help of a global clinical score. Patients were also asked to assess their skin condition for hydration, roughness, brown spots, glare, fine wrinkles and coarse wrinkles with the help of a grading scale (1 = much improved, 2 = somewhat improved, 3 = no change, 4 = somewhat worse and 5 = much worse).
Cirteria Assessed
Efficacy
Primary Endpoint
- Mean change in the global clinical score for photodamage at weeks 4, 8 and 12 from the baseline.
Secondary Endpoint
- Mean change in patients' self-assessment at weeks 4, 8 and 12 from the baseline.
- Change in photographic assessment at week 12 from the baseline.
Safety
- Skin tolerability.
- Incidence and nature of adverse events.
- Incidence of drug-related adverse reactions.
Results
Out of the 22 patients enrolled in the study, 20 completed the study and 2 patients discontinued as they did not return for the follow-up visit.
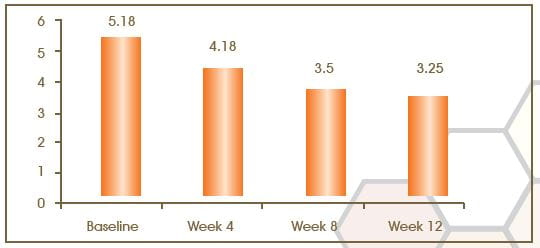
Improvement in the Global Clinical Score for Photodamage
The global clinical score at baseline was 5.18 ± 1.30 and reduced to 4.18 ± 1.01 at week 4 of the treatment period, with a mean change of 1.00 (p=0.000). At week 12 of the treatment period, the score was 3.25 ± 0.79 with a mean change of 1.70 (p=0.000).
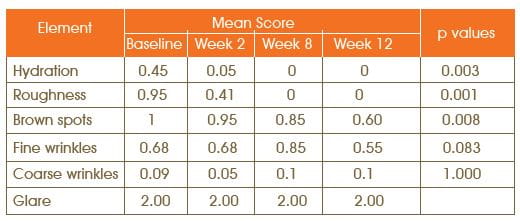
Significant change was seen in the score for hydration (p=0.003), roughness (p=0.001) and brown spots (p=0.008). At the beginning of the study, 9 out of 10 patients were graded as having drier than normal skin and all improved to normal hydration at week 4 of the evaluation. Skin roughness symptoms completely resolved in 5 patients with mild symptoms, and in all the 7 patients with moderate roughness, it changed to mild roughness. The symptoms of dryness and roughness were absent in all patients within 8 weeks of treatment.
Mean Change in the Subject's Self-Assessment Score from Baseline to Weeks 4, 8 and 12
By 8 weeks of treatment period, 8 (40%) patients reported much improvement in hydration and 5 (25%) patients reported somewhat improvement in hydration. After 8 weeks of therapy, 10 (50%) of patients reported much improvement in roughness. Regarding brown spots, 5 (25%) patients reported much improvement and 10 (50%) patients reported somewhat improvement by 12 weeks of treatment.
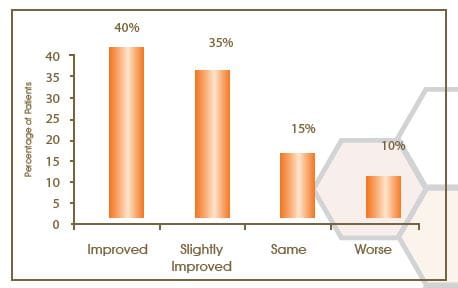
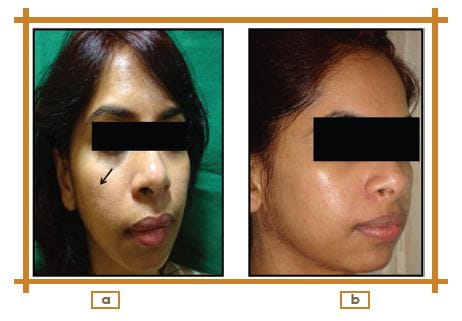
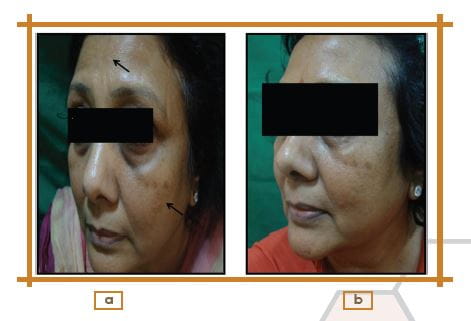
Change in Photographic Assessment at Week 12 from the Baseline
Photographic assessment showed an improvement in 40%, slight improvement in 35%, no change in 15%, and worsening in 10% of patients.
Conclusion
The results of the study showed that the Vitamin C 15% serum formulation is effective, safe and well-tolerated for the treatment of patients with photodamaged skin. The improvement was apparent by 8 weeks of therapy.
Data on File, Cipla Ltd.