This study was an open-label, non-comparative, single-centre study to assess the efficacy and safety of Vitamin C 15% serum in melasma.
Evaluation of the Efficacy and Safety of Topical Vitamin C 15% Serum in Melasma
Objective
Methodology
The study schedule consisted of five visits at an interval of every 4 weeks. In this study, 22 patients of age 18 years and above were screened for inclusion and exclusion criteria after obtaining a written informed consent.
At the screening visit, the patients were assessed using demography, complete medical and surgical history, vital signs and skin examination, and with the help of the Melasma Area and Severity Index (MASI) score. From visit 2 onwards and at every subsequent visit, the investigator asked the patients to assess the change in the melasma patch in the following categories of lightening: less than 25% (Mild); 25-50% (Moderate); 50-<75% (Good); and >75% (excellent), compared to the start of the treatment. Photographic assessments were done pre- and post-treatment. Follow-up of the patients were done at weeks 4, 8, 12 and 16 from the baseline visit.
Patients who satisfied the selection criteria were enrolled into 16 weeks of treatment period. These patients received one bottle of Vitamin C 15% serum for the treatment of melasma. The patients were advised to apply 7 drops of Vitamin C 15% once daily on the face at morning.
During the study, the patients were allowed to use sunscreen lotion. The sunscreen lotion was to be used three times a day; but it was advised that it should not be applied within 1 hour of the application of the study medication. Patients were instructed not to apply any cosmetics on the day of the clinical assessment. Adverse events, previous and concomitant medications were assessed at each visit.
Criteria Assessed
Efficacy
Primary Endpoint
- Improvements in the MASI score from baseline to 4, 8, 12 and 16 weeks of the treatment period.
Secondary Endpoint
- Results of patient's self-assessment from baseline to 4, 8, 12 and 16 weeks.
- Photographic improvement from baseline to 16 weeks.
Safety
- Skin tolerability.
- Incidence and nature of adverse events.
- Incidence of drug related adverse reactions
Results
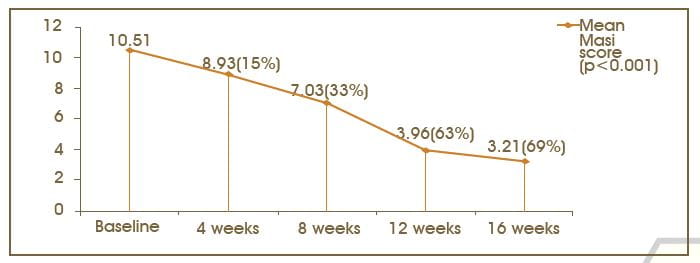
MASI Score: Visit 1 to Visit 5
The objective assessment for the drug was performed, based on the MASI score. The mean MASI score at the baseline visit was 10.42 ± 4.80. The score after 4 and 8 weeks was 8.93 ± 4.30 and 7.03 ± 3.65, respectively; this was further reduced to 3.96 - 3.66 and 3.21 ± 3.64 after weeks 12 and 16, respectively. Statistically significant reduction (p<.001; 95% confidence interval [CI]), in the mean MASI score was observed from the baseline visit to week 16.
The MASI score reduced from 10.42 ± 4.80 to 3.21 ± 3.66, showing a 69% reduction in 16 weeks, which was statistically significant. The reduction in the MASI score was rapid at every visit until 12 weeks. The reduction in the MASI score was statistically significant at every visit from the baseline.
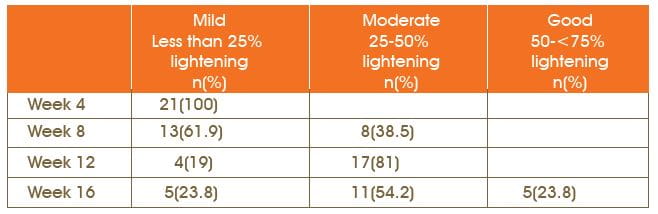
Patients' Self-Assessment of Lightening of Melasma Patch
At every visit, the subjective response was assessed, based on the lightening of the melasma patch after 4 weeks of drug application. After using the drug for the first 4 weeks, all the patients had reported mild improvement, i.e. (less than 25% lightening in the melasma patch). After 16 weeks of treatment at the last visit, 5 (23.8%) patients reported the improvement as good (50-<75% lightening of the melasma patch), 11 (54.2%) patients reported moderate improvement (25-50% lightening of the melasma patch) and 5 (23.8%) patients reported mild improvement (less than 25% lightening of the melasma patch). Statistically significant changes (p<0.001; 95% CI) were observed in lightening of the patch from visit 2 to visit 5.
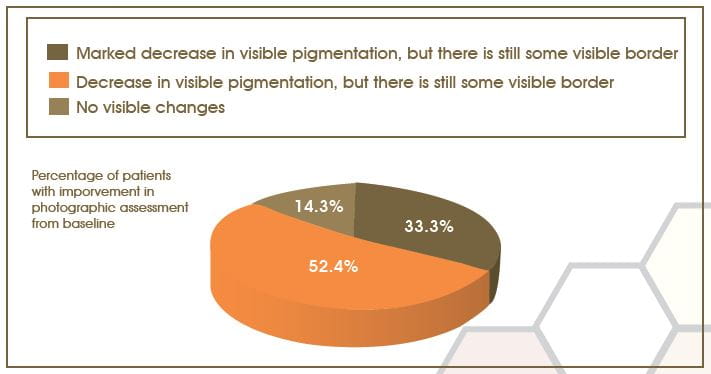
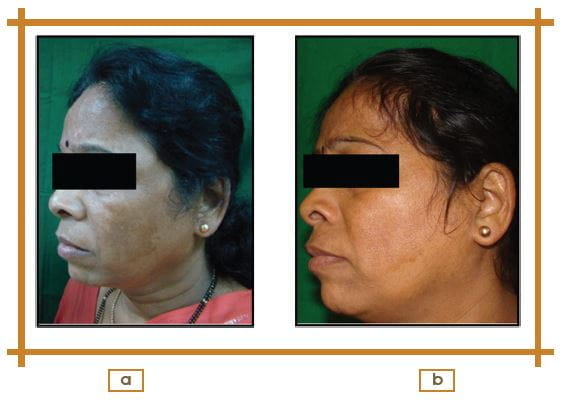
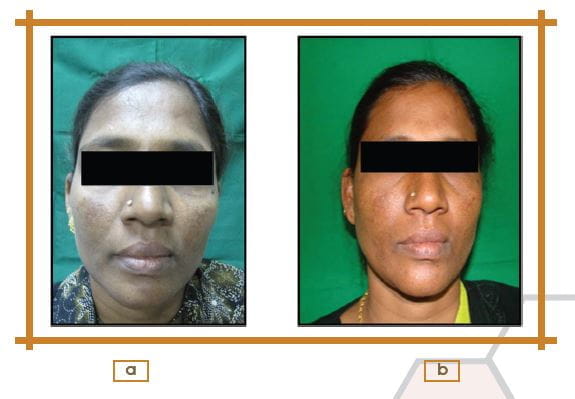
Photographic Assessment from Baseline to 16 Weeks
According to photographic assessment, 33.30% patients showed a marked decrease of visible pigmentation with some visible border, and 52.40% patients showed decrease in visible pigmentation with some visible borders; however no visible change were seen in 14.30% of patients.
Statistically significant change was observed (p<0.001; 95% CI), in pre- and post-treatment assessment of the photographs.
Conclusion
A daily, 4-month regimen of topical Vitamin C 15% serum showed objective as well as subjective improvement in melasma. From the study results, we can conclude that, Vitamin C 15% serum was an effective, safe and well-tolerated treatment for melasma. The improvement was apparent by 8 weeks of therapy.
Dat on file, Cipla Ltd.