Global Epidemiology of HIV/AIDS
More people than ever are living with the human immunodeficiency virus (HIV), largely due to greater access to treatment. The UNAIDS 2016 Global AIDS updates, report shows that tremendous efforts and a strategic approach has succeeded in dramatically improving the HIV scenario today. The highlights are as follows:
People Living with HIV1
- In 2015, there were 36.7 million people living with HIV.
New HIV Infections1
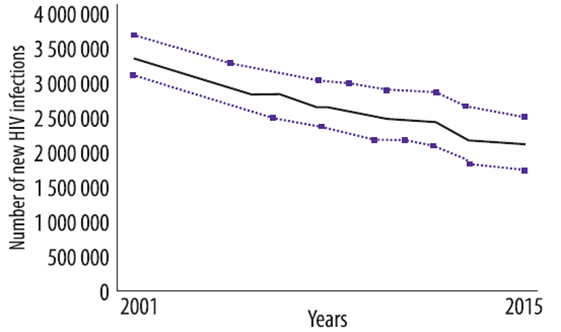
- Worldwide, 2.1 million people became newly infected with HIV in 2015.
- A 38% decline in the number of new infections from 3.4 million since 2001.
AIDS-related Deaths1
- In 2015, 1.1 million people died from AIDS-related causes worldwide.
- AIDS-related deaths have fallen by 43% since 2003.
Access to Antiretroviral Therapy (ART)
- Percentage of HIV-positive people not receiving ART has reduced from 90% in 2006 to 63% in 2013.2
- Currently there are 17 million people globally receiving ART at the end of 2015.1
From 1995 to 2013, ART has averted 7.6 million AIDS-related deaths globally.2
HIV/TB2
- From 2004 to 2012, tuberculosis (TB)-related deaths among people living with HIV declined by 36% worldwide.
Prevention of Mother-to-child Transmission (PMTCT)2
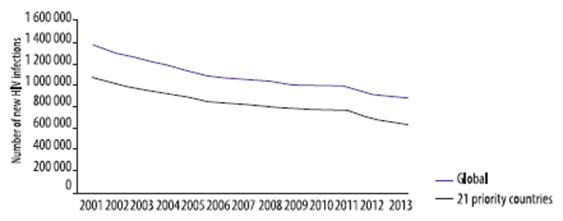
- In 2013, 240,000 children were newly infected with HIV. This was 58% lower than in 2002, the year with the highest number (580,000) became newly infected with HIV.
- Providing access to antiretrovirals (ARVs) for pregnant women living with HIV has averted more than 900,000 new HIV infections among children since 2009.
Reference
- Global AIDS update, UNAIDS 2016
- UNAIDS 2015 Gap report on HIV/AIDS
Introduction to Antiretroviral Therapy (ART)
Significant advances have been made in our understanding of the pathogenesis and progression of HIV disease. These include development of effective ARV drugs and regimens, learning how best to use these regimens for maximal and prolonged viral suppression, reducing the pill burden and managing various adverse events occurring during treatment.1 ART has already resulted in life expectancy gains of over 11 years in hyper-epidemic settings.2
Goals of ART3
- To achieve maximal and durable virologic suppression (ideally, a viral load <50 copies/ml)
- To reconstitute and preserve immunologic function
- To reduce morbidity and mortality
- To improve the quality of life
- To prevent transmission of HIV
Additionally, ARV drugs can be used to reduce the transmission of HIV in various situations, as listed below:
- Prevention of transmission of HIV from an infected mother to her child.
- From a HIV-positive partner to a HIV-negative partner (serodiscordant couple).
- For post-exposure prophylaxis (PEP) after occupational exposures and nonoccupational exposures.
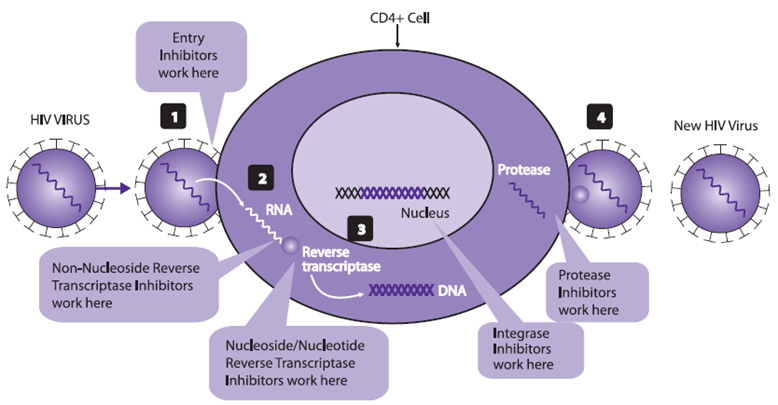
Classes of ARVs
Currently, the following six classes of ARV agents are approved by the USFDA for use in HIV-infected patients.
|
Nucleoside reverse transcriptase inhibitors (NRTIs) |
Non-Nucleoside reverse transcriptase inhibitors (NNRTIs) |
Protease inhibitors (PIs) |
Fusion inhibitors |
Integrase inhibitors (INSTIs) |
CCR5 co- receptor antagonists |
|
Abacavir (ABC) |
Delavirdine (DLV) |
Atazanavir (ATV) |
Enfuvirtide (T20) |
Dolutegravir (DTG) |
Maraviroc (MVC) |
|
Didanosine (ddI) |
Efavirenz (EFV) |
Darunavir (DRV) |
|
Elvitegravir (EVG) |
|
|
Emtricitabine (FTC) |
Etravirine (ETR) |
Fosamprenavir (FPV) |
|
Raltegravir (RAL) |
|
|
Lamivudine (3TC) |
Nevirapine (NVP) |
Indinavir (IDV) |
|
|
|
|
Stavudine (d4T) |
Rilpivirine (RIL) |
Nelfinavir (NFV) |
|
|
|
|
Tenofovir disoproxil fumarate (TDF) |
|
Ritonavir (RTV) |
|
|
|
|
Zidovudine (AZT, ZDV) |
|
Saquinavir (SQV) |
|
|
|
|
Tipranavir (TPV) |
|||||
|
Lopinavir (LPV) |
Rationale for Combination Therapy
HIV has the ability to rapidly develop resistance if any one drug is used alone. Thus, in order for HIV therapy to be effective and durable, a minimum of three drugs have to be used in combination. This triple-drug regimen is commonly referred to as HAART, which is an acronym for highly active ART.
Strategies for Using ART
Even if triple therapy is used, there is a possibility, over a period of time, of the virus developing resistance and the patient failing his/her initial treatment regimen. Hence, the initiation of ART should be viewed as the beginning of a long-term strategy. When choosing an initial regimen, possible future combinations have to be planned, keeping in mind issues of resistance and cross-resistance among ARVs.
References
- Venkataramana Kandi. HIV Patient Care: An Overview N Management of Complications Arising from Highly Active Antiretroviral Therapy (HAART), J Pat Care 2016; 2:110.
- Jacob Bor, Abraham J Herbst. Increases in adult life expectancy in rural South Africa: valuing the scale-up of HIV treatment. Science 2013; 339:961-965.
- Consolidated Guidelines on the Use Of Antiretroviral Drugs For Treating And Preventing HIV Infection, November 2015.
- Antiretroviral drugs used in the treatment of HIV infection, U. S. FDA
Initiating Antiretroviral Therapy (ART)
The new World Health Organization (WHO) recommendations (2016) on when to start ART now support ART initiation in all adults, adolescents and children with HIV at any CD4 cell count or disease stage. Further, the guidelines recommend that efforts should be made to reduce the time between diagnosis and ART initiation to improve health outcomes.
|
Preparing people living with HIV for ART |
|
|
Accelerated ART Initiation Good practice statement |
Efforts should be made to reduce the time between HIV diagnosis and ART initiation based on an assessment of a person’s readiness. |
|
When to start ART |
|
|
When to start ART in adults (>19 years old) |
ART should be initiated in all adults living with HIV, regardless of WHO clinical stage and at any CD4 cell count (strong recommendation, moderate-quality evidence). |
|
As a priority, ART should be initiated in all adults with severe or advanced HIV clinical disease (WHO clinical stage 3 or 4) and adults with CD4 count ≤350 cells/mm3 (strong recommendation, moderate-quality evidence). |
|
|
When to start ART in adolescents (10–19 years of age) |
ART should be initiated in all adolescents living with HIV, regardless of WHO clinical stage and at any CD4 cell count (conditional recommendation, low-quality evidence).
As a priority, ART should be initiated in all adolescents with severe or advanced HIV clinical disease (WHO clinical stage 3 or 4) and adolescents with CD4 count ≤350 cells/mm3 (strong recommendation, moderate-quality evidence) |
What to Start (first-line regimens)1?
The WHO encourages the use of fixed-dose combinations and once-daily dosing regimens.
|
|
Adults |
Adolescents |
|
Preferred regimens |
TDF + 3TC (or FTC) + EFV |
TDF + 3TC (or FTC) + EFV |
|
Alternative regimens |
AZT + 3TC + EFV (or NVP) TDF + 3TC (or FTC) + DTGa TDF+3TC (or FTC)+EFV400a TDF + 3TC (or FTC) + NVP |
TDF (or ABC) + 3TC (or FTC) + DTGa TDF (or ABC) + 3TC (or FTC) + EFV 400a ABC + 3TC + EFV (or NVP) ABC + FTC + NVP AZT + 3TC + EFV (or NVP) TDF + 3TC (or FTC) + NVP |
|
Special circumstances b,c |
Regimens containing ABC and boosted PIs |
Regimens containing boosted PIs |
a To date, there is limited experience with the use of low-dose EFV and DTG in pregnant, breastfeeding women and adolescents. While no age or weight restrictions apply to the use of EFV 400 mg/day, which can be used starting from a weight of 20 kg, the use of DTG is approved only for adolescents who are older than 12 years and weigh more than 40 kg.
b Special circumstances may include situations where preferred or alternative regimens may not be available or suitable because of significant toxicities, anticipated drug–drug interactions, drug procurement and supply management issues, or for other reasons.
c Using stavudine (d4T) as an option in first-line treatment should be discontinued.
3TC lamivudine, ABC abacavir, ATV atazanavir, AZT zidovudine, DTG dolutegravir, EFV efavirenz, FTC emtricitabine, NVP nevirapine, PI protease inhibitor, TDF tenofovir.
Points to be Discussed Prior to Initiating ART
There are many issues associated with the use of ART. It is critical that patients be adequately counselled on the following points, prior to initiating therapy:
- Commitment to lifelong therapy to avoid emergence of drug resistance.
- Affordability and access to ART for an indefinite period.
- High level of adherence is a crucial determinant of therapeutic success with ARVs.
- Short-term and long-term adverse events associated with ART may occur.
- Drug interactions may occur with concomitantly prescribed drugs.
- Counselling for safer sex practices is important to prevent HIV transmission.
Reference
- WHO, Consolidated Guidelines on the Use of Antiretroviral Drugs For Treating And Preventing HIV Infection, Second Edition, 2016
Monitoring the Patient on ART
|
Phase of HIV management |
Recommended |
Desirable (if feasible) |
|
HIV diagnosis |
HIV testing (serology for adults and children 18 months or older; EID for children younger than 18 months) CD4 cell count TB symptom screening |
HBV (HBsAG) serologya HCV serology Cryptococcus antigen if CD4 count <100 cells/mm3b Screening for STIs Pregnancy test to assess if ART initiation should be prioritized to prevent HIV transmission to the child Assessment for major noncommunicable chronic diseases and comorbiditiesc |
|
Follow-up before ART |
CD4 cell count (every 6–12 months in circumstances where ART initiation is delayed) |
|
|
ART initiation |
|
Haemoglobin test for starting AZTd Pregnancy test Blood pressure measurement Serum creatinine and estimated glomerular filtration rate (eGFR) or starting TDFe Alanine aminotransferase for NVPf Baseline CD4 cell count |
|
Receiving ART |
HIV viral load (at 6 months and 12 months after initiating ART and every 12 months thereafter) CD4 cell count every 6 months until patients are stable on ART |
Serum creatinine and eGFR for TDFc Pregnancy test, especially for women of childbearing age not receiving family planning and on treatment with DTG or low-dose EFV |
a If feasible, HBsAg testing should be performed at baseline to identify people with HIV and HBV coinfection and who should therefore initiate TDF-containing ART.
b Can be considered in settings with a high prevalence of cryptococcal antigenaemia (>3%).
c Consider assessing for the presence of chronic conditions that can influence ART management, such as hypertension and other cardiovascular diseases, diabetes and TB according to the WHO Package of Essential NCD interventions (PEN), mental health Gap Action Programme (mhGAP) or national standard protocols. Monitoring may include a range of tests, including serum creatinine and estimated glomerular filtration rate (eGFR), serum phosphate and urine dipsticks for proteinuria and glycosuria.
d Among children and adults with a high risk of adverse events associated with AZT (low CD4 or low BMI).
e Among people with a high risk of adverse events associated with TDF: underlying renal disease, older age group, low body mass index (BMI), diabetes, hypertension and concomitant use of a boosted PI or potential nephrotoxic drugs.
f Among people with a high risk of adverse events associated with NVP, such as being ART-naive, women with HIV with a CD4 count >250 cells/mm3 and hepatitis C virus (HCV) coinfection. However, liver enzymes have low predictive value for monitoring NVP toxicity.
ART antiretroviral therapy, AZT zidovudine, EID early infant diagnosis, HBV hepatitis B virus, HBsAg hepatitis B surface antigen, HCV hepatitis C virus, STI sexually transmitted infection, TDF tenofovir.
The WHO guidelines (November 2015) recommend viral load be used as the preferred monitoring approach to diagnose and confirm treatment failure. New recommendations encourage routine viral load testing performed 6 and 12 months after initiating ART and, if the patient is stable on ART, every 12 months thereafter.1
The WHO (2015) supports stopping routine CD4 count testing where viral load testing is available, in individuals who are stable on ART and virally suppressed.1
Less frequent clinical visits (3–6 months) are recommended by the WHO (2016) for people stable on ART.1
Reference
- WHO, Consolidated Guidelines on the Use of Antiretroviral Drugs For Treating And Preventing HIV Infection, Second Edition, 2016
When to Change the First-line Regimen?
The first-line regimen may need to be modified for one of the following reasons:
- Due to emergence of toxicity to one of the components of the first-line regimen. This is referred to as substitution.
- Due to failure of the first-line treatment. This is referred to as switching.
These are further discussed below.
Substitution1
After initiating the first-line regimen, it may be necessary to substitute one or more drugs in the triple regimen due to the following reasons:
- Toxicity (e.g. TDF for AZT anaemia)
- Simplification (e.g. b.i.d. to q.d. regimens)
- Cost
- Drug-drug interactions (e.g. from NVP to EFV when initiating rifampicin)
- Pregnancy
- Proactive (e.g. from d4t to TDF)
Substitution should be carried out only after confirming virologic suppression.
|
ARV Drug |
Major types of toxicity |
Risk factors |
Suggested management |
|
ABC |
Hypersensitivity reaction |
Presence of HLA-B*5701 allele |
Do not use ABC in the presence of HLA-B*5701 allele. Substitute with AZT or TDF. |
|
ATV/r |
Electrocardiographic abnormalities (PR and QRS interval prolongation) |
People with pre-existing conduction system disease Concomitant use of other drugs that may prolong the PR or QRS intervals Congenital long QT syndrome |
Use with caution in people with pre- existing conduction disease or who are on concomitant drugs that may prolong the PR or QRS intervals. |
|
Indirect hyperbilirubinaemia (clinical jaundice) |
Presence of uridine diphosphate (UDP)- glucuronosyltransferase 1A1*28 (UGT1A1*28) allele |
This phenomenon is clinically benign but potentially stigmatizing. Substitute only if adherence is compromised. |
|
|
Nephrolithiasis |
History of nephrolithiasis |
Substitute with LPV/r or DRV/r. If boosted PIs are contraindicated and NNRTIs have failed in first-line ART, consider substituting with integrase inhibitors. |
|
|
AZT |
Severe anaemia, neutropaenia |
CD4 cell count of ≤200 cells/ mm3 |
Substitute with TDF or ABC. Consider use of low-dose zidovudine. |
|
Lactic acidosis or severe hepatomegaly with steatosis, lipoatrophy, lipodystrophy, myopathy |
BMI >25 (or body weight >75 kg) Prolonged exposure to NRTIs |
Substitute with TDF or ABC. |
|
|
DTG |
Hepatotoxicity Hypersensitivity reactions |
Hepatitis B or C coinfection Liver disease |
If DTG is used in first-line ART, and there are hypersensitivity reactions, substitute with another therapeutic class (EFV or boosted PIs). |
|
DRV/r |
Hepatotoxicity |
Underlying hepatic disease HBV and HCV coinfection Concomitant use of hepatotoxic drugs |
Substitute with ATV/r or LPV/r. When it is used in third-line ART, limited options are available. For hypersensitivity reactions, substitute with another therapeutic class. |
|
Severe skin and hypersensitivity reactions |
Sulfonamide allergy |
||
|
EFV |
Persistent central nervous system toxicity (such as dizziness, insomnia, abnormal dreams) or mental symptoms (anxiety, depression, mental confusion) |
Depression or other mental disorder (previous or at baseline) |
For CNS symptoms, dose at night- time. Consider using EFV at a lower dose (400 mg/day) or substitute with NVP or integrase inhibitor (DTG) if EFV 400 mg is not effective in reducing symptoms. For severe hepatotoxicity or hypersensitivity reactions, substitute with another therapeutic class (integrase inhibitors or boosted PIs). |
|
Hepatotoxicity |
Underlying hepatic disease HBV and HCV coinfection Concomitant use of hepatotoxic drug |
||
|
Convulsions |
Histor of seizure |
||
|
Severe skin and hypersensitivity reactions |
Risk factor(s) unknown |
||
|
EFV |
Gynaecomastia |
Risk factor(s) unknown |
Substitute with NVP or another therapeutic class (integrase inhibitors or boosted PIs). |
|
ETV |
Severe skin and hypersensitivity reactions |
Risk factor(s) unknown |
Substitute with another therapeutic class (integrase inhibitors or boosted PIs). |
|
LPV/r |
Electrocardiographic abnormalities (PR and QRS interval prolongation, torsades de pointes) |
People with pre-existing conduction system disease Concomitant use of other drugs that may prolong the PR or QRS intervals Congenital long QT syndrome Hypokalaemia |
Use with caution in people with pre- existing conduction disease or those on concomitant drugs that may prolong the PR or QRS intervals. |
|
Hepatotoxicity |
Underlying hepatic disease HBV and HCV coinfection Concomitant use of hepatotoxic drugs |
If LPV/r is used in first-line ART for children, substitute with NVP or RAL for children younger than 3 years and EFV for children 3 years and older. ATV can be used for children older than 6 years.
If LPV/r is used in second-line ART for adults, and the person has treatment failure with NNRTI in first-line ART, consider integrase inhibitors. |
|
|
Dyslipidaemia |
Cardiovascular risk factors such as obesity and diabetes |
Substitute with another therapeutic class (integrase inhibitors). |
|
|
Pancreatitis |
Advanced HIV disease, alcohol misuse |
|
|
|
Diarrhoea |
|
Substitute with ATV/r, DRV/r or integrase inhibitors. |
|
|
NVP |
Hepatotoxicity Severe skin rash and hypersensitivity reaction, including Stevens- Johnson syndrome |
Underlying hepatic disease HBV and HCV coinfection Concomitant use of hepatotoxic drugs High baseline CD4 cell count (CD4 count >250 cells/mm3 in women or >400 cells/mm3 in men) |
If hepatotoxicity is mild, consider substitution with EFV, including in children 3 years and older. For severe hepatotoxicity and hypersensitivity, and in children under the age of 3 years, substitute with another therapeutic class (integrase inhibitors or boosted PIs). |
|
RAL |
Rhabdomyolysis, myopathy, myalgia |
Concomitant use of other drugs that increase the risk of myopathy and rhabdomyolysis, including statins |
Substitute with another therapeutic class (etravirine, boosted PIs). |
|
|
Hepatitis and hepatic failure Severe skin rash and hypersensitivity reaction |
Risk factors unknown |
|
|
TDF |
Chronic kidney disease Acute kidney injury and Fanconi syndrome |
Underlying renal disease Older than 50 years of age BMI <18.5 or low body weight (<50 kg) notably in females Untreated diabetes Untreated hypertension Concomitant use of nephrotoxic drugs or a boosted PI |
Substitute with AZT or ABC. Do not initiate TDF at eGFR <50 mL/ min, uncontrolled hypertension, untreated diabetes, or presence of renal failure. |
|
Decreases in bone mineral density |
History of osteomalacia (in adults) and rickets (in children) and pathological fracture Risk factors for osteoporosis or bone mineral density loss Vitamin D deficiency |
||
|
Lactic acidosis or severe hepatomegaly with steatosis |
Prolonged exposure to nucleoside analogues Obesity Liver disease |
ABC abacavir, ATV atazanavir, AZT zidovudine, CNS central nervous system, DRV darunavir, DTG dolutegravir, EFV efavirenz, eGFR estimated glomerular filtration rate, HBV hepatitis B virus, HCV hepatitis C virus, LPV lopinavir, NNRTI non-nucleoside reverse transcriptase inhibitor, NVP nevirapine, PI protease inhibitor, r ritonavir, RAL raltegravir, TDF tenofovir.
Switching
When the first-line regimen fails, the patient should be initiated on a second-line regimen.
|
Failure |
Adults and adolescents |
|
Clinical failure |
New or recurrent clinical event indicating severe immunodeficiency (WHO clinical stage 4 condition) after 6 months of effective treatment |
|
Immunological failure |
CD4 count at or below 250 cells/mm3 following clinical failure or Persistent CD4 levels <100 cells/mm3 |
|
Virological failure |
Plasma viral load >1,000 copies/ml based on two consecutive viral load measurements after 3 months, with adherence support |
|
Viral load is recommended as the preferred monitoring approach to diagnose and confirm treatment failurea (strong recommendation, low-quality evidence). |
|
If viral load is not routinely available, CD4 count and clinical monitoring should be used to diagnose treatment failure (strong recommendation, moderate-quality evidence). |
|
Dried blood spot specimens using venous or capillary whole blood can be used to determine HIV viral load. A threshold of 1,000 copies/mL can be used to determine virological failure when using dried blood spot samples, as defined for testing in plasma (conditional recommendation, low-quality evidence) |
a Plasma specimens are preferred for viral load testing. Dried blood spot specimens are recommended for use in settings where logistical, infrastructural or operational barriers prevent routine viral load monitoring using plasma specimens.
Once failure is detected, identifying the cause of failure is important before modifying the regimen. Points like adherence, drug–drug interactions and continuing highrisk behaviour need to be assessed. Once resistance is suspected, a second-line ART regimen should be designed for the patient.
Recommended second- and third-line ART regimens for adults and adolescents
The WHO has issued recommendations for preferred second-line regimens and alternatives, in line with ART optimizing principles, availability of fixed-dose combinations, tolerability and the risk of resistant mutations.
The principles for constructing third-line regimens is to include new drugs with minimal risk of cross-resistance to previously used regimens, and patients on a failing second-line regimen with no new ARV options should continue with a tolerated regimen.
|
Population |
First-line regimen |
Second-line regimen |
Third-line regimen |
|
Adults and Adolescents (> 10 years) |
2 NRTIs + EFV |
2 NRTIs + ATV/r or LPV/ra |
DRV/rb + DTGc (or RAL) ± 1–2 NRTIs |
|
2 NRTIs + DRV/rb |
|||
|
2 NRTIs + DTG |
2 NRTIs + ATV/r or LPV/r |
DRV/rb + 2 NRTIs ± NNRTI |
|
|
2 NRTIs + DRV/r |
Optimize regimen using genotype profile |
a RAL + LPV/r can be used as an alternative second-line regimen in adults and adolescents.
b In PI-experienced patients, the recommended DRV/r dose should be 600 mg/100 mg twice daily.
c Safety and efficacy data on the use of DTG in adolescents younger than 12 years and pregnant women are not yet available.
ATV atazanavir, DRV darunavir, DTG dolutegravir, EFV efavirenz, LPV lopinavir, NNRTI non-nucleoside reverse transcriptase inhibitor, NRTI nucleoside reverse-transcriptase inhibitor, r ritonavir, RAL raltegravir.
References
- Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection, June 2013
- WHO, Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection, WHO, Second Edition, 2016
Managing HIV/TB Co-infection
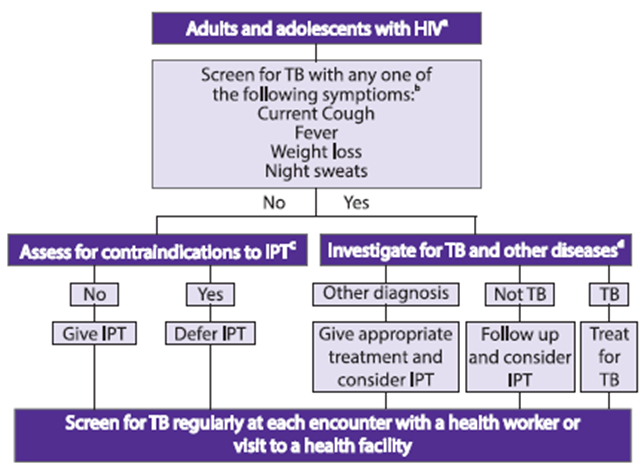
Tuberculosis (TB) is the most frequent life-threatening opportunistic infection in HIV and a leading cause of death. Adults and adolescents living with HIV should be screened for TB with a clinical algorithm; those who report any one of the symptoms of current cough, fever, weight loss or night sweats may have active TB and should be evaluated for TB and other diseases.
IPT: isoniazid preventive therapy
a Every adult and adolescent should be evaluated for eligibility to receive antiretroviral therapy. Infection control measures should be given priority to reduce Mycobacterium tuberculosis transmission in all settings that provide care.
b Chest radiography can be done if available but is not required to classify people into TB and non-TB groups. In settings with high HIV prevalence and a high TB prevalence among people living with HIV (such as exceeding 10%), strong consideration must be given to adding other sensitive investigations.
c Contraindications include; active hepatitis (acute or chronic), regular and heavy alcohol consumption and symptoms of peripheral neuropathy. Past history of TB and current pregnancy should not be contraindications for starting isoniazid preventive therapy. Although not a requirement for initiating isoniazid preventive therapy, tuberculin skin testing may be performed as a part of eligibility screening in some settings.
d Investigations for TB should be performed in accordance with existing national guidelines.
Treatment of TB in HIV-Infected patients
The basic principles of TB treatment in HIV-infected individuals are the same as for HIV uninfected individuals. The standard therapeutic regimen for TB consists of isoniazid (INH), a rifamycin such as rifampicin (RIF) or rifabutin (RFB), pyrazinamide (PZA), and ethambutol (EMB) given for 2 months, followed by INH plus a rifamycin for 4 months.
The optimal duration of anti-TB therapy is based on the risk of relapse, and current guidelines recommend the same duration of treatment, regardless of HIV status.
|
|
First-line ART for Adults with TB coinfection |
First-line ART for Adolescents with TB coinfection a,b |
|
Preferred regimens |
TDF + 3TC (or FTC) + EFV |
Two NRTIs + EFV or
Triple NRTI (AZT + 3TC + ABC)c |
|
Alternative regimens |
AZT + 3TC + EFV (or NVP) TDF + 3TC (or FTC) + NVP |
a Ensure optimal dosing of rifampicin based on dosing guidelines.
b Substitute ARV drugs based on an age-appropriate ART regimen in line with nationally recommended first-line ART.
c Triple NRTI is only recommended for the duration of TB treatment; an age-appropriate PI- or NNRTI-based regimen should be restarted when rifampicin-based therapy ends. Based on the findings from the ARROW trial, this regimen should be considered as the preferred option for children younger than 3 years who are receiving an LPV/r-based regimen when starting TB treatment. The US FDA approval for the use of EFV in children 3 months to 3 years old weighing more than 3.5 kg offers a potential alternative to the triple-NRTI approach. An EFV-based regimen in children under 3 years is still not recommended because pharmacokinetic data are needed to ensure that the co-administration of rifampicin does not decrease drug levels below the therapeutic level. Triple NRTI should also be considered as the preferred regimen for children older than 3 years with a history of failure on an NNRTI-based regimen.
TDF Tenofovir, 3TC lamivudine, ABC abacavir, AZT zidovudine, EFV efavirenz, FTC emtricitabine, NRTI nucleoside reverse transcriptase inhibitor, NVP nevirapine.
It is currently recommended that co-infected individuals receive prompt treatment for both diseases, regardless of CD4+ cell count. Treatment of active TB infection is the priority due to the risk of transmitting TB to other people, however, when CD4+ T cell count is extremely low (<50 cells/mm3), prompt initiation of ART is necessary. Recommendations of the WHO guidelines for when to initiate ART in HIV–TB coinfected patients is given in the table below:1,2
|
Timing of ART for adults with TB |
ART should be started in all TB patients living with HIV regardless of CD4 count (strong recommendation, high-quality evidence).
TB treatment should be initiated first, followed by ART as soon as possible within the first 8 weeks of treatment (strong recommnendation,high-quality evidence).
HIV-positive T patients with profound immunosuppressin (eg .CD4 counts less than 50 cells/mm3 should receive ART within the first two weeks of initaiating TB treatment. |
Advantages of early HAART administration include higher cure rates, reduced risk of relapse, reduced risk of infection with other HIV-associated opportunistic infections, and lower mortality rates. Several studies showed that early initiation of ART decreased mortality for HIV-associated TB. Randomized controlled trials such as SAPiT, CAMELIA and STRIDE have suggested and provided evidence for immediate institution of ART within 2 weeks of starting anti- mycobacterial therapy.3,4
Complications in the Management of HIV-TB Co-infection
Management of HIV-TB co-infection is complicated due to several factors such as potential drug interactions, cumulative toxicity, therapeutic failure, non-compliance due to pill burden, and the risk of immune reconstitution inflammatory syndrome (IRIS).5
- Drug–drug interactions
Drug–drug interactions between HIV-1 and TB therapy are common. Rifampicin is the most powerful inducer of CYP3A4, which is the main enzyme responsible for the metabolism of PIs and NNRTIs. Rifampicin can be administered with EFV.3,5
RIF significantly reduces plasma concentrations of PIs and, therefore, cannot be coadministered safely at standard dosages. When it is necessary to give a rifamycinbased TB treatment with RTV-boosted PIs, the alternate is either to use RFB rather than RIF, or using RIF with increased dosages of RTV or the PI.
- TB- Immune Reconstitution Inflammatory Syndrome (TB-IRIS)
TB-IRIS is a common early complication for patients receiving HIV/TB treatment simultaneously. The condition is thought to result from the recovering immune system driving inflammatory reactions directed at the M. tuberculosis antigen present at sites of disease. TB-IRIS is characterized by excessive local or systemic inflammation. Anti-inflammatory drugs and steroids are the mainstay therapy for IRIS. Discontinuation of HAART is not warranted in most cases. Delaying initiation of ART for 2–8 weeks may reduce the incidence and severity of IRIS.5,6
References
- WHO, Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach, June 2013
- WHO, Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach, Second Edition, 2016.
- C. Schutz, G. Meintjes et al; Clinical management of tuberculosis and HIV-1 co-infection, Eur Respir J 2010; 36: 1460–1481. DOI: 10.1183/09031936.00110210
- M Kendon, S E Knight, et al; Timing of antiretroviral therapy initiation in adults with HIV-associated tuberculosis: Outcomes of therapy in an urban hospital in KwaZulu-Natal, S Afr Med J 2012; 102(12):931-935. DOI:10.7196/SAMJ.5574
- Maria Theresa Montales, Arun Chaudhury et al; Hiv-Associated TB Syndemic: A Growing Clinical Challenge worldwide, Front. Public Health, 2015; 3:281. doi: 10.3389/fpubh. 2015.00281
- Annie Luetkemeyer; Tuberculosis and HIV, HIV InSite Knowledge Base Chapter January 2013; http://hivinsite.ucsf.edu/InSite?page=kb-05-01-06#S9X, Last Accessed May 2016
Prevention of Mother-to-Child Transmission
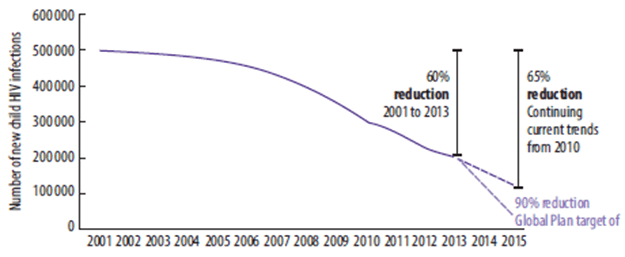
In 2013, about 1.3 million women living with HIV gave birth, a figure that is unchanged from 2009. However, the number of children newly infected fell from 350,000 in 2009 to 199,000 in 2013. The rate of mother-to-child transmission also fell in 2013; 16% of children born to women living with HIV became infected compared to 25.8% in 2009.1
In pregnant and breastfeeding women with HIV, ARV drugs are used for both the mother’s health and to prevent the exposed child from becoming infected.
When to Start ART in Pregnant and Breastfeeding Women
All pregnant and breastfeeding women with HIV should initiate ART, regardless of WHO clinical stage and at any CD4 count, and this should be continued lifelong.2
|
First-line ART |
Preferred first-line regimen |
Alternative first-line regimena,b |
|
Pregnant/breastfeeding women |
TDF + 3TC (or FTC) + EFV |
AZT + 3TC + EFV (or NVP) TDF + 3TC (or FTC) + NVP |
a For adults and adolescents, d4T should be discontinued as an option in first-line treatment.
b ABC or boosted PIs (ATV/r, DRV/r, LPV/r) can be used in special circumstances.
|
Population |
First-line regimen |
Second-line regimen |
Third-line regimen |
|
Pregnant/ breastfeeding women |
2 NRTIs + EFV |
2 NRTIs + ATV/r or LPV/ra |
DRV/rb + DTGc (or RAL) ± 1-2 NRTIs |
|
2 NRTIs + DRV/rb |
a RAL + LPV/r can be used as an alternative second-line regimen in adults and adolescents.
b In PI-experienced patients, the recommended DRV/r dose should be 600 mg/100 mg twice daily.
c Safety and efficacy data on the use of DTG in adolescents younger than 12 years and pregnant women are not yet available.
ATV atazanavir, DRV darunavir, DTG dolutegravir, EFV efavirenz, LPV lopinavir, NRTI nucleoside reverse-transcriptase inhibitor, NVP nevirapine, PI protease inhibitor, r ritonavir, RAL raltegravir.
|
Infant prophylaxis |
Infants born to mothers with HIV who are at high risk of acquiring HIV* should receive dual prophylaxis with AZT (twice daily) and NVP (once daily) for the first 6 weeks of life, whether they are breastfed or formula-fed (strong recommendation, moderate-quality evidence). |
|
Breastfed infants who are at high risk of acquiring HIV* including those first identified as exposed to HIV during the postpartum period, should continue infant prophylaxis for an additional 6 weeks (total of 12 weeks of infant prophylaxis) using either AZT (twice daily) and NVP (once daily) or NVP alone (conditional recommendation, low-quality evidence). |
|
|
Infants of mothers who are receiving ART and are breastfeeding should receive 6 weeks of infant prophylaxis with daily NVP. If infants are receiving replacement feeding, they should be given 4 to 6 weeks of infant prophylaxis with daily NVP (or twice-daily AZT) (strong recommendation, moderate quality evidence for breastfeeding infants; strong recommendation, low-quality evidence for infants receiving only replacement feeding). |
|
|
Good practice statement |
ART should be initiated urgently in all pregnant and breastfeeding women, even if they are identified late in pregnancy or postpartum, because the most effective way to prevent mother-to-child HIV transmission is to reduce maternal viral load. |
* High-risk infants are defined as those:
- born to women with established HIV infection who have received less than 4 weeks of ART at the time of delivery, OR
- born to women with established HIV infection with viral load >1000 copies/mL in the 4 weeks before delivery, if viral load measurements available, OR
- born to women with incident HIV infection during pregnancy or breastfeeding, OR
- identified for the first time during the postpartum period, with or without a negative HIV test prenatally.
References
- UNAIDS Gap report, 2015
- WHO, Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection, second Edition, 2016.
Treating Paediatric HIV
At the end of 2013, 240,000 children were newly infected with HIV. This is 58% lower than in 2002, the year when the highest number (580,000) became newly infected with HIV. However, ART coverage still lags signifi cantly among children compared to adults.1
Infants and young children have an exceptionally high risk of poor outcomes from HIV infection. Up to 52% of children die before the age of 2 years in the absence of any intervention. By 5 years of age, the risk of mortality and disease progression in the absence of treatment falls to rates similar to those of young adults.1
|
Criteria |
Preferred starting time for ART |
|
All children living with HIV |
ART should be initiated in all children living with HIV, regardless of WHO clinical stage or at any CD4 cell count. |
|
Infants <1 year |
ART should be initiated in all infants diagnosed in the first year of life (strong recommendation, moderate-quality evidence). |
|
Children 1 year old to less than 10 years old |
ART should be initiated in all children living with HIV 1 year old to less than 10 years old (conditional recommendation, low-quality evidence). |
|
Priority ART |
ART should be initiated in all children ≤2 years of age or children younger than 5 years of age with WHO HIV clinical stage 3 or 4 or CD4 count ≤750 cells/mm³ or CD percentage <25%, and children 5 years of age and older with WHO HIV clinical stage 3 or 4 disease or CD4 count ≤350 cells/mm3 (strong recommendations, moderate-quality evidence) |
What to start in ART for children?
Once-daily regimens comprising a NRTI backbone (TDF + FTC or TDF + 3TC) and one NNRTI (EFV) are maintained as the preferred choices in children older than 3 years of age. For children younger than 3 years of age, a PI-based regimen is the preferred approach.
|
|
Children 3-10 years of age |
Children below 3 years of age |
|
Preferred regimen |
ABC + 3TC + EFV |
ABCa or AZT + 3TC + LPV/rb |
|
Alternative regimenc |
ABC + 3TC + NVP AZT + 3TC + EFV (or NVP) TDF + 3TC (or FTC) + EFV (or NVP) |
ABCa or AZT + 3TC + NVP |
|
Special considerationd |
- |
ABCa or AZT + 3TC + RALe |
a Based on the general principle of using non-thymidine analogues in first-line regimens and thymidine analogues in second-line regimens, ABC should be considered as the preferred NRTI whenever possible. Availability and cost should be carefully considered
b As recommended by the US FDA, using LPV/r oral liquid should be avoided in premature babies (born 1 month or more before the expected date of delivery) until 14 days after their due date or in full-term babies younger than 14 days of age. Dosing for children younger than 6 weeks should be calculated based on body surface area. Restrictions also apply to LPV/r pellets, where administration challenges extend to infants up to 3 months of age. Additional information regarding optimal administration of this formulation will be provided as more data become available.
c In children below 3 years of age: Challenges may arise when treatment is started in the first two weeks of life following early diagnosis at or around birth, particularly in case of prematurity or low birth weight. In these situations, an NVP-based regimen containing AZT and 3TC should be started, and NVP should be substituted with LPV/r at the earliest opportunity, preferably at two weeks when LPV/r syrup can be administered. In settings where LPV/r syrup is not available and LPV/r pellets are the only formulation available, administration of NVP should continue until 3 months with close clinical monitoring for those children considered at high risk for carrying NNRTI resistance (i.e. prolonged NVP-based postnatal prophylaxis or documented NRTI failure in the mother).
d Special circumstances may include situations where preferred or alternative regimens may not be available or suitable because of significant toxicities, anticipated drug–drug interactions, drug procurement and supply management issues or for other reasons.
e RAL is approved for use in infants and children from the age of 4 weeks, but there is very limited evidence to inform the use of raltegravir (RAL) as a fi rst-line drug in infants and young children. The use of this INSTI could be considered where available in instances of poor tolerability or administration challenges with LPV/r, particularly in settings where as a result of rapid expansion of maternal treatment, infants and children are at very high risk of carrying an NNRTI resistance virus. Use of RAL should however consider the challenges of existing granule formulation, despite being suitable for use in infants 4 weeks and older, as reconstitution in water is required before administration. While dispersion of RAL chewable tablets is considered to be a potential alternative, additional information regarding the appropriateness of this approach will be provided as more data become available.
3TC lamivudine, ABC abacavir, AZT zidovudine, EFV efavirenz, FTC emtricitabine, LPV lopinavir, NVP Nevirapine, r ritonavir, RAL raltegravir, TDF tenofovir.
|
|
Failing first-line regimen |
Preferred second- line regimen |
Alternative second- line regimen |
Third-line regimen |
|
Children 3-10 years of age |
2 NRTIs + LPV/ra |
2 NRTIsb + EFV |
2 NRTIsb + RALc |
RAL (or DTG)d + 2 NRTIs DRV/re + 2 NRTIs DRV/re + RAL (or DTG)d ± 1–2 NRTIs |
|
2 NRTIs + EFV (or NVP) |
2 NRTIsb + LPV/r |
2 NRTIsb + ATV/rc |
||
|
Children below 3 years of age |
2 NRTIs + LPV/r |
2 NRTIsb + RAL |
Maintain the failing LPV/r-based regimen and switch to 2 NRTIsb + EFV at 3 years of age |
|
|
2 NRTIs + NVP |
2 NRTIsb + LPV/r |
2 NRTIsb + RALc |
a ATV/r can be used as an alternative PI for children older than 3 months of age.
b If ABC+ 3TC or TDF + 3TC (or FTC) was used in the first-line failing regimen, AZT + 3TC should be used in second-line and vice versa.
c DRV/r can be used as an alternative PI option in special situations.
d RAL can be used in children failing PI-based second-line treatment when DTG is not available and when RAL has not been not used in a previous regimen. DTG is currently approved only for children 12 years and older; however, studies are ongoing to determine dosing in younger children, and approval for lower age groups is expected in the near future
e DRV/r should not be used in children younger than 3 years of age.
ATV atazanavir, DRV darunavir, DTG dolutegravir, EFV efavirenz, LPV lopinavir, NVP nevirapine, r or RTV ritonavir, RAL Raltegravir, NRTI nucleoside reverse-transcriptase inhibitor
References
- UNAIDS, Gap report, 2015
- WHO, Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection, Second Edition, 2016.
Post-Exposure Prophylaxis
During the past two decades, HIV post-exposure prophylaxis (PEP) has been extended to non-occupational exposures, including unprotected sexual exposure, injecting drug use and exposure following sexual assault.1
Evidence supporting the use of ARV drugs for PEP comes from animal studies and a single case control study in healthcare workers that demonstrated that ARV drugs could prevent the establishment of chronic HIV infection if administered within a short time following exposure.1
Standard of Care1
Everyone possibly exposed to HIV should be assessed by a trained healthcare worker. Essential components of the clinical pathway include assessing the mechanism of exposure and assessing eligibility for PEP, examination of any wound and initial first-aid treatment.
Figure 6: Care pathway for people exposed to HIV1
|
Practical guidance |
|
• Post-exposure prophylaxis should be offered, and initiated as early as possible, to all individuals with exposure that has the potential for HIV transmission, preferably within 72 hours.a |
|
• Assessment for eligibility should be based on the HIV status of the source whenever possible and may include consideration of background prevalence and local epidemiological patterns.b – parenteral or mucosal membrane exposure (sexual exposure and splashes in the eye, nose or oral cavity); and – the following bodily fluids may pose a risk of HIV infection: blood, blood-stained saliva, breast-milk, genital secretions and cerebrospinal, amniotic, rectal, peritoneal, synovial, pericardial or pleural fluids.c |
|
• Exposures that does not require post-exposure prophylaxis include: – when the exposed individual is already HIV positive; – when the source is established to be HIV negative; and – exposure to bodily fluids that does not pose a significant risk: tears, non-blood-stained saliva, urine and sweat |
a Although post-exposure prophylaxis is ideally provided within 72 hours of exposure, people may not be able to access services within this time. Providers should consider the range of other essential interventions and referrals that should be offered to clients presenting after the 72 hours.
b In some settings with high background HIV prevalence or where the source is known to be high risk for HIV infection, all exposure may be considered for post-exposure prophylaxis without risk assessment.
c These fluids carry a high risk of HIV infection, but this list is not exhaustive and all cases should be assessed clinically and decisions made by the healthcare workers as to whether exposure constitutes significant risk.
Assessment of Exposed Person’s HIV status1
HIV testing should be performed using rapid diagnostic tests that can provide definitive results in most cases within 2 hours and often within 20 minutes.
Assessment of the Source Person’s HIV Status1
HIV testing of the source person should be conducted to guide appropriate clinical action and inform the exposed individual and, where possible, the source of their HIV status.
In settings with generalized HIV epidemics, it is reasonable to assume that all sources of unknown HIV status may pose a risk of infection.
- If the source is determined to be HIV-positive, provision should be made to link them to appropriate treatment and care.
- If the source is established to be HIV-negative, PEP should be discontinued.
Duration of PEP1
- A 28-day prescription of antiretroviral drugs should be provided for HIV post-exposure prophylaxis following initial risk assessment (strong recommendation, low-quality evidence).2
- Enhanced adherence counselling (includes baseline individual needs assessment, adherence counselling and education sessions and follow-up telephone calls) is suggested for individuals initiating HIV post-exposure prophylaxis (conditional recommendation, moderate-quality evidence).2
In accordance with ART guidance, trained non-physicians, midwives, nurses and other non-clinical health providers can initiate and dispense ARV drugs for PEP.1
Individuals should be aware of the risks and benefits of PEP, and verbal consent should be sought.1
Everyone should be informed of potential drug–drug interactions and possible side effects and toxicity. Promoting adherence is critical to improving completion rates, which are generally low in most populations and settings.
Preferred PEP ARV Regimens for Adults and Adolescents
The latest WHO guidelines for ART, issued in 2016, give preference to tenofovir disoproxil fumarate (TDF) and lamivudine (3TC) or emtricitabine (FTC) as a backbone for first-line treatment for adults and adolescents. Harmonization of ART regimens for adults and children is recommended whenever possible.2
|
TDF + 3TC (or FTC) is recommended as the preferred backbone regimena for HIV PEP for adults and adolescents. (Strong recommendation, low-quality evidence) |
|
LPV/r or ATV/r is recommended as the preferred third drug for HIV PEP for adults and adolescents. (Conditional recommendation, very-low-quality evidence) |
|
Where available, RAL, DRV/r or EFV can be considered as alternative options. |
a Backbone regimen refers to the two-NRTI component of an ART regimen (normally comprising of 3 ARV drugs)
|
Generic name |
Dose |
|
Tenofovir (TDF) |
300 mg once daily |
|
Lamivudine (3TC) |
150 mg twice daily or 300 mg once daily |
|
Emtricitabine (FTC) |
200 mg once daily |
|
Lopinavir/ritonavir (LPV/r) |
400 mg/100 mg twice daily or 800 mg/200 mg once dailya |
|
Atazanavir/ritonavir (ATV/r) |
300 mg + 100 mg once daily |
|
Raltegravir (RAL) |
400 mg twice daily |
|
Darunavir + ritonavir (DRV/r) |
800 mg + 100 mg once daily or 600 mg + 100 mg twice daily |
|
Efavirenz (EFV) |
600 mg once daily |
|
|
|
a Once-daily dosing can be considered as an alternative for adults, but more data are needed for children and adolescents.
References
- Guidelines on post-exposure prophylaxis for HIV, December 2014
- WHO, Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection, Second Edition, 2016.
Pre-Exposure Prophylaxis (PrEP)
Oral PrEP is the use of antiretroviral (ARV) drugs before HIV exposure by people who are not infected with HIV in order to block the acquisition of HIV.
WHO recommendations1
The WHO recommends that oral PrEP containing TDF should be offered as an additional prevention choice for people at substantial risk of HIV infection as part of combination HIV prevention approaches. (strong recommendation, high-quality evidence)
Substantial risk of HIV infection is defined by an incidence of HIV infection in the absence of PrEP that is suffi ciently high (>3% incidence) to make offering PrEP potentially cost-saving (or cost-effective).
Combination HIV prevention refers to a combination of behavioural, biomedical and structural approaches to HIV prevention to achieve maximum impact on reducing HIV transmission and acquisition.
Rationale and supporting evidence
Clinical trials of daily oral PrEP have shown evidence of effectiveness with serodiscordant heterosexual couples,2 men and transgender women who have sex with men,3 high-risk heterosexual couples4 and people who inject drugs.5
A systematic review and meta-analysis of PrEP trials containing TDF demonstrated that PrEP is effective in reducing the risk of acquiring HIV infection.6 The level of protection did not differ by age, gender, regimen (TDF versus FTC + TDF) and mode of acquiring HIV (rectal, penile or vaginal). The level of protection was strongly correlated with adherence.
|
|
Men who have sex with men |
Heterosexual women and men |
Injection drug users |
|
Detecting substantial risk of acquiring HIV infection |
HIV-positive sexual partner Recent bacterial STI High number of sex partners History of inconsistent or no condom use Commercial sex work |
HIV-positive sexual partner Recent bacterial STI High number of sex partners History of inconsistent or no condom use Commercial sex work In high-prevalence area or network |
HIV-positive injecting partner Sharing injection equipment Recent drug treatment (but currently injecting) |
|
Clinically eligible |
Documented negative HIV test result before prescribing PrEP No signs/symptoms of acute HIV infection Normal renal function; no contraindicated medications Documented hepatitis B virus infection and vaccination status |
||
|
Prescription |
Daily, continuing, oral doses of TDF/FTC, ≤90-day supply |
||
|
Other services |
Follow-up visits at least every 3 months to provide the following: HIV test, medication adherence counselling, behavioural risk reduction support, side effect assessment, STI symptom assessment. At 3 months and every 6 months thereafter, assess renal function. Every 6 months, test for bacterial STIs. |
||
|
Do oral/rectal STI testing |
Assess pregnancy intent Pregnancy test every 3 months |
Access to clean needles/syringes and drug treatment services |
|
STI=sexually transmitted infection
References
- WHO, Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection, Second Edition, 2016.
- Baeten JM, Donnell D et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012; 367(5): 399-410.
- Grant RM, Lama JR et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010 Dec 30; 363(27): 2587-99.
- Thigpen MC, Kebaabetswe PM et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012 Aug 2; 367(5): 423-34.
- Choopanya K, Martin M et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2013 Jun; 381(9883): 2083-90.
- Fonner, Virginia A. Eff ectiveness and safety of oral HIV pre-exposure prophylaxis (PrEP) for all populations: A systematic review and meta-analysis. AIDS. 2016 May doi: 10.1097/QAD.0000000000001145.
- US Public Health Service. Pre-exposure Prophylaxis for the Prevention of HIV Infection in the United States – 2014 Clinical Practice Guideline
Treatment as Prevention (TasP)
Serodiscordant couples are couples in which one partner is living with HIV and the other partner is HIV-negative. Thus, the negative partner is at a high risk of acquiring HIV.1
ART has been eff ective in reducing the amount of HIV-1 in genital secretions. Because the sexual transmission of HIV-1 from infected persons to their uninfected partners is strongly correlated with concentrations of HIV-1 in blood and in the genital tract, it has hypothesized that ART could reduce sexual transmission of the virus. Several observational studies have reported decreased acquisition of HIV-1 by HIV-negative sexual partners of patients receiving ART.2
The HPTN 052 study randomised the positive partner in heterosexual couples, either to start taking ART immediately, at an average CD4 count of 436 cells/mm3, or to delay taking ART till their CD4 count fell below 250 cells/mm3. The study found that people on ART were 96% less likely to transmit HIV to their partners than untreated people.3
Thus, the HPTN 052 shows that, if a suffi ciently large proportion of the HIV-positive population could be treated and their viral loads brought down to an undetectable level, transmission might be prevented.3
The WHO recommends that partners with HIV in serodiscordant couples should be offered ART at any CD4 count to reduce HIV transmission to uninfected partners.1
References
- WHO guidelines on when to start ART and on PrEP for HIV, September 2015
- Consolidated guidelines on HIV prevention, diagnosis, treatment and care for key populations, July 2014
- Myron S. Cohen, Ying Q. Chen et al., Prevention of HIV-1 Infection with Early Antiretroviral Therapy, N Engl J Med 2011; 365:493-505.
Appendix–1: Overview of ARVs
|
NRTIs (Nucleoside reverse transcriptase inhibitors) |
||
|
Drug |
Dosage and administration |
Side effects |
|
Abacavir (ABC) |
600 mg once daily or 300 mg twice daily Take without regard to meals. |
• Hypersensitivity reactions (HSRs): Patients who test positive for HLA-B*5701 are at highest risk. HLA screening should be done before initiation of ABC. Rechallenge is not recommended. • Symptoms of HSR may include fever, rash, nausea, vomiting, diarrhoea, abdominal pain, malaise, fatigue or respiratory symp- toms such as sore throat, cough, or short- ness of breath. • Some cohort studies suggest increased risk of myocardial infarction (MI) with recent or current use of ABC, but this risk is not substantiated in other studies. |
|
Didanosine (ddI) |
Body weight > 60 kg: 400 mg once daily Body weight <60 kg: 250 mg once daily Take on empty stomach (half- hour before or 2 hours after a meal). |
• Pancreatitis • Peripheral neuropathy • Retinal changes, optic neuritis • Lactic acidosis with hepatic steatosis with/ without pancreatitis (rare but potentially life-threatening toxicity). • Nausea, vomiting • Potential association with non-cirrhotic portal hypertension, in some cases, patients presented with oesophageal varices. • One cohort study suggested increased risk of MI with recent or current use of ddI, but this risk is not substantiated in other studies • Insulin resistance/diabetes mellitus. |
|
Emtricitabine (FTC) |
200 mg once daily Take without regard to meals. |
• Minimal toxicity • Hyperpigmentation/skin discolouration • Severe acute exacerbation of hepatitis may occur in HBV co-infected patients who dis- continue FTC |
|
Lamivudine (3TC) |
150 mg twice daily or 300 mg once daily Take without regard to meals. |
• Minimal toxicity. • Severe acute exacerbation of hepatitis may occur in HBV co-infected patients who dis- continue 3TC. |
|
Stavudine (d4T) |
Body weight >60 kg: 40 mg twice daily Body weight <60 kg: 30 mg twice daily Take without regard to meals. WHO recommends 30 mg twice daily dosing regardless of body weight |
• Peripheral neuropathy • Lipoatrophy • Pancreatitis • Lactic acidosis/severe hepatomegaly with hepatic steatosis (rare but potentially life- threatening toxicity). • Hyperlipidaemia • Insulin resistance/diabetes mellitus • Rapidly progressive ascending neuromuscu- lar weakness (rare). |
||||||||||
|
Tenofovir disoproxil fu- marate (TDF) |
300 mg once daily Take without regard to meals. |
• Renal insufficiency, Fanconi syndrome, proximal renal tubulopathy. • Osteomalacia, decrease in bone mineral density. • Severe acute exacerbation of hepatitis may occur in HBV co-infected patients who dis- continue TDF. • Asthenia, headache, diarrhoea, nausea, vomiting, and flatulence. |
||||||||||
|
Zidovudine (ZDV or AZT) |
300 mg twice daily Take without regard to meals. |
• Bone marrow suppression: macrocytic anae- mia or neutropenia. • Nausea, vomiting, headache, insomnia, as- thenia. • Nail pigmentation • Lactic acidosis/severe hepatomegaly with hepatic steatosis (rare but potentially life- threatening toxicity). • Hyperlipidaemia • Insulin resistance/diabetes mellitus • Lipoatrophy • Myopathy |
||||||||||
|
NNRTIs (Non-nucleoside reverse transcriptase inhibitors) |
|
|||||||||||
|
Drug |
Dosage and administration |
Side effects |
|
|||||||||
|
Efavirenz (EFV) |
600 mg once daily Take on an empty stomach, preferably at bedtime. |
• Rash • Neuropsychiatric symptoms • Increased transaminase levels • Hyperlipidaemia • False-positive results with some cannabinoid and benzodiazepine screening assays reported. • Teratogenic in nonhuman primates and potentially teratogenic during the first trimester in humans. |
|
|||||||||
|
Etravirine (ETR)* |
200 mg twice daily Take following a meal. |
• Rash, including Stevens-Johnson syndrome. • HSRs, characterized by rash, constitutional findings, and sometimes organ dysfunction (including hepatic failure) have been reported. • Nausea |
|
|||||||||
|
Nevirapine (NVP 200 mg) Or Nevirapine XR (NVP 400 mg)* |
200 mg once daily for 14 days (lead-in period); Thereafter, 200 mg (NVP) twice daily or 400 mg (NVP XR) once daily Take without regard to meals. |
• Rash, including Stevens-Johnson syndrome. • Symptomatic hepatitis, including fatal hepatic necrosis, has been reported: – rash reported in approximately 50% of cases; – occurs at significantly higher frequency in ARV-naïve female patients with pre- NVP CD4 counts >250 cells/mm3 and in ARV-naïve male patients with pre-NVP CD4 counts >400 cells/mm3. NVP should not be initiated in these patients unless the benefit clearly outweighs the risk. |
|
|||||||||
|
Rilpivirine (RPV)* |
25 mg once daily Take with a meal. |
• Rash • Depression, insomnia, headache • Hepatotoxicity |
|
|||||||||
|
PIs (Protease Inhibitors)
|
|
|||||||||||
|
Drug |
Dosage and administration |
Side effects |
|
|||||||||
|
Atazanavir (ATV) |
ARV-naïve patients: ATV 400 mg once daily or (ATV 300 mg + RTV 100 mg) once daily ARV-experienced patients: (ATV 300 mg + RTV 100 mg) once daily Take with a meal. |
• Indirect hyperbilirubinaemia. • PR interval prolongation: First-degree symptomatic AV block reported. Use with caution in patients with underlying conduction defects or on concomitant medications that can cause PR prolongation. • Hyperglycaemia • Fat maldistribution • Cholelithiasis • Nephrolithiasis • Renal insufficiency • Serum transaminase elevations • Hyperlipidaemia (especially with RTV boosting) • Skin rash • Increase in serum creatinine (with COBI) |
|
|||||||||
|
Darunavir (DRV) |
ARV-naïve patients or ARV- experienced patients with no DRV mutations: (DRV 800 mg + RTV 100 mg) once daily ARV-experienced patients with at least one DRV mutation: (DRV 600 mg + RTV 100 mg) twice daily Unboosted DRV is not recommended Take with a meal. |
• Skin rash (10%): DRV has a sulphonamide moiety; Stevens-Johnson syndrome, toxic epidermal necrolysis, acute generalized exanthematous pustulosis, and erythrema multiforme have been reported. • Hepatotoxicity • Diarrhoea, nausea • Headache • Hyperlipidaemia • Serum transaminase elevation • Hyperglycaemia
• Fat maldistribution • Increase in serum creatinine (with COBI) |
|
|||||||||
|
Fosamprenavir (FPV)* |
ARV-naïve patients: FPV 1,400 mg twice daily or (FPV 1,400 mg + RTV 100–200 mg) once daily or (FPV 700 mg + RTV 100 mg) twice daily PI-experienced patients (once-daily dosing not recommended): (FPV 700 mg + RTV 100 mg) twice daily With EFV: (FPV 700 mg + RTV 100 mg) twice daily or (FPV 1,400 mg + RTV 300 mg) once daily Take without regard to meals (if not boosted with RTV tablet) Take with a meal if boosted with RTV. |
• Skin rash (12–19%): FPV has a sulphonamide moiety • Diarrhoea, nausea, vomiting • Headache • Hyperlipidaemia • Serum transaminase elevation • Hyperglycaemia • Fat maldistribution • Possible increased bleeding episodes in patients with haemophilia • Nephrolithiasis |
|
|||||||||
|
Indinavir (IDV) |
800 mg every 8 hours Take 1 hour before or 2 hours after meals. With RTV: (IDV 800 mg + RTV 100–200 mg) twice daily Take without regard to meals. |
• Nephrolithiasis • GI intolerance, nausea • Hepatitis • Indirect hyperbilirubinaemia • Hyperlipidaemia • Headache, asthenia, blurred vision, dizziness, rash, metallic taste, thrombocytopaenia, alopecia, and haemolytic anaemia • Hyperglycaemia • Fat maldistribution • Possible increased bleeding episodes in patients with haemophilia |
|
|||||||||
|
Lopinavir (LPV) |
(LPV 400 mg + RTV 100 mg) twice daily or (LPV 800 mg + RTV 200 mg) once daily Once-daily dosing is not recommended for patients with ≥3 LPV-associated mutations, pregnant women, or patients receiving EFV, NVP, FPV, NFV, carbamazepine, phenytoin, or phenobarbital. PI-naïve or PI-experienced patients: (LPV 500 mg + RTV 125 mg) twice daily Take without regard to meals. |
• GI intolerance, nausea, vomiting, diarrhoea • Pancreatitis • Asthenia • Hyperlipidaemia (especially hypertriglyceridaemia) • Serum transaminase elevation • Hyperglycaemia • Insulin resistance/diabetes mellitus • Fat maldistribution • Possible increased bleeding episodes in patients with haemophilia • PR interval prolongation • QT interval prolongation and torsades de pointes have been reported; however, causality could not be established. |
|
|||||||||
|
Nelfinavir (NFV) |
1,250 mg twice daily or 750 mg thrice daily Take with a meal. |
• Diarrhoea • Hyperlipidaemia • Hyperglycaemia • Fat maldistribution • Possible increased bleeding episodes in patients with haemophilia • Serum transaminase elevation |
|
|||||||||
|
Ritonavir (RTV) |
As pharmacokinetic booster for other PIs: 100–400 mg per day in one to two divided doses (refer to other PIs for specific dosing recommendations) Take with a meal. |
• GI intolerance, nausea, vomiting, diarrhoea • Paraesthesias (circumoral and extremities) • Hyperlipidaemia (especially hypertriglyceridaemia) • Hepatitis • Asthenia • Taste perversion • Hyperglycaemia • Fat maldistribution • Possible increased bleeding episodes in patients with haemophilia. |
|
|||||||||
|
Saquinavir (SQV) |
(SQV 1,000 mg + RTV 100 mg) twice daily Unboosted SQV in not recommended Take with a meal or within 2 hours after a meal |
• GI intolerance, nausea, and diarrhoea • Headache • Serum transaminase elevation • Hyperlipidaemia • Hyperglycaemia • Fat maldistribution • Possible increased bleeding episodes in patients with haemophilia • PR interval prolongation • QT interval prolongation, torsades de pointes have been reported. Patients with pre-SQV QT interval >450 msec should not receive SQV. |
|
|||||||||
|
Tipranavir (TPV)* |
(TPV 500 mg + RTV 200 mg) twice daily Unboosted TPV is not recommended Take with a meal. |
• Hepatotoxicity: Clinical hepatitis (including hepatic decompensation and hepatitis-associated fatalities) has been reported; monitor patients closely, especially those with underlying liver diseases. • Skin rash (3–21%): TPV has a sulphonamide moiety; use with caution in patients with known sulphonamide allergy. • Rare cases of fatal and nonfatal intracranial haemorrhages have been reported. Risks include brain lesion, head trauma, recent neurosurgery, coagulopathy, hypertension, alcoholism, and the use of anti-coagulant or anti-platelet agents (including vitamin E). • Hyperlipidaemia • Hyperglycaemia • Fat maldistribution • Possible increased bleeding episodes in patients with haemophilia |
|
|||||||||
|
INSTIs (Integrase Inhibitors) |
|
|||||||||||
|
Drug |
Dosage and administration
|
Side effects |
|
|||||||||
|
Raltegravir (RAL) |
400 mg twice daily With rifampicin: 800 mg twice daily Take without regard to meals. |
• Rash, including Stevens-Johnson syndrome, HSR and toxic epidermal necrolysis • Nausea • Headache • Diarrhoea • Pyrexia • CPK elevation, muscle weakness, and rhabdomyolysis • Insomnia |
|
|||||||||
|
Dolutegravir (DTG)* |
ARV-naïve or ARV- experienced, INSTI-naïve patients: 50 mg once daily ARV-naïve or ARV- experienced, INSTI-naïve patients when co- administered with EFV, FPV/r, TPV/r, or RIF: 50 mg twice daily INSTI-experienced patients with certain INSTI mutations (see product label) or with clinically suspected INSTI resistance: 50 mg twice daily Take without regard to meals. |
• HSRs, including rash, constitutional symptoms and organ dysfunction (including liver injury) have been reported. • Insomnia • Headache |
|
|||||||||
|
Elvitegravir (EVG)* |
With once-daily ATV/r or twice-daily LPV/r: 85 mg once daily with food With twice-daily DRV/r, FPV/r, or TPV/r: 150 mg once daily with food Unboosted EVG is not recommended |
• Nausea • Diarrhoea |
|
|||||||||
|
Fusion Inhibitors |
|
|||||||||||
|
Drug |
Dosage and administration |
Side effects |
|
|||||||||
|
Enfuvirtide (T20)* |
90 mg (1 mL) subcutaneously twice daily |
• Local injection site reactions (e.g. pain, erythema, induration, nodules and cysts, pruritus, ecchymosis) in almost 100% of patients, • Increased incidence of bacterial pneumonia, • HSR (<1% of patients): Symptoms may include rash, fever, nausea, vomiting, chills, rigors, hypotension, or elevated serum transaminases. Re-challenge is not recommended. |
|
|||||||||
|
CCR5 Antagonist |
|
|||||||||||
|
Drug |
Dosage and administration |
Side effects |
|
|||||||||
|
Maraviroc (MVC)* |
150 mg twice daily when given with drugs that are strong CYP3A inhibitors (with or without CYP3A inducers), including PIs (except TPV/r) 300 mg twice daily when given with NRTIs, T20, TPV/r, NVP, RAL, and other drugs that are not strong CYP3A inhibitors or inducers 600 mg twice daily when given with drugs that are CYP3A inducers, including EFV, ETR, etc. (without a CYP3A inhibitor) Take without regard to meals. |
• Abdominal pain • Cough • Dizziness • Musculoskeletal symptoms • Pyrexia • Rash • Upper respiratory tract infections • Hepatotoxicity, which may be preceded by severe rash or other signs of systemic allergic reactions • Orthostatic hypotension, especially in patients with severe renal insufficiency |
|
|||||||||
Appendix–2: Cipla’s Range of WHO and USFDA Approved Antiretrovirals for Adults and Paediatrics
|
WHO Pre-qualified |
USFDA Approved |
|
Abacavir 300 mg tablets |
Nevirapine 200 mg tablets |
|
Efavirenz 200 mg capsules |
Efavirenz 600 mg tablets |
|
Efavirenz 600 mg tablets |
Abacavir Sulfate 300 mg tablets |
|
Emtricitabine 200 mg capsules |
Tenofovir Disoproxil Furmarate 300 mg tablets |
|
Lamivudine 300 mg tablets |
Zidovudine 300 mg tablets |
|
Nevirapine 200 mg tablets |
Emtricitabine 200 mg capsules |
|
Ritonavir 100 mg tablets |
Ritonavir Tablets 100 mg tablets |
|
Tenofovir disoproxil fumarate 300 mg tablets |
Nevirapine 400 mg extended release tablets |
|
Zidovudine 300 mg tablets |
Lamivudine / Zidovudine 150 / 300 mg tablets |
|
Abacavir (as sulfate) / Lamivudine 600 / 300 mg tablets |
Lopinavir / Ritonavir 200 / 50 mg tablets |
|
Emtricitabine / Tenofovir disoproxil fumarate 200 / 300 mg tablets |
Lamivudine / Tenofovir Disoproxil Fumarate 300 / 300 mg tablets |
|
Lamivudine / Tenofovir disoproxil fumarate 300 / 300 mg tablets |
Abacavir Sulfate / Lamivudine 600 / 300 mg tablets |
|
Lopinavir / Ritonavir 200 / 50 mg tablets |
Emtricitabine / Tenofovir Disoproxil Fumarate 200 / 300 mg tablets |
|
Efavirenz / Emtricitabine / Tenofovir disoproxil fumarate 600 / 200 / 300 mg tablets |
Lamivudine / Zidovudine / Nevirapine 150 / 300 / 200 mg tablets |
|
Efavirenz / Lamivudine / Tenofovir disoproxil fumarate 600 / 300 / 300 mg tablets |
Efavirenz / Emtricitabine / Tenofovir Disoproxil Fumarate 600 / 200 / 300 mg tablets |
|
Lamivudine / Nevirapine / Zidovudine 150 / 200 / 300 mg Film-coated tablets |
Efavirenz / Lamivudine / Tenofovir Disoproxil Fumarate 600 / 300 / 300 mg tablets |
|
WHO Pre-qualified |
USFDA Approved |
|
Lamivudine 50 mg / 5 ml oral solution |
Zidovudine 50 mg / 5 ml oral solution |
|
Zidovudine 50 mg / 5 ml oral solution |
Lamivudine 10 mg / ml oral solution |
|
Nevirapine 50 mg / 5 ml oral suspension |
Abacavir 20 mg / ml oral solution |
|
Abacavir 20 mg / ml oral solution |
Lopinavir / Ritonavir 80 / 20 mg / ml oral solution |
|
Abacavir 60 mg tablets for oral suspension |
Ritonavir 25 / 50 mg tablets |
|
Lamivudine 30 mg tablets |
Abacavir / Lamivudine 60 mg / 30 mg tablets for oral solution |
|
Nevirapine 50 / 100 mg dispersible tablets |
Lamivudine / Zidovudine 30 / 60 mg tablets |
|
Abacavir / Lamivudine 60 mg / 30 mg dispersible tablets |
Lamivudine / Zidovudine 30 / 60 mg tablets for oral solution |
|
|
Nevirapine 50 / 100 mg tablets for oral solution |
|
|
Lopinavir / Ritonavir 40 / 10 mg oral pellets |
Appendix–3
|
Clinical stage 1 |
|
• Asymptomatic • Persistent generalized lymphadenopathy |
|
Clinical stage 2 |
|
• Moderate unexplained weight loss (<10% of presumed or measured body weight) • Recurrent respiratory tract infections (sinusitis, tonsillitis, otitis media, pharyngitis) • Herpes zoster • Angular cheilitis • Recurrent oral ulceration • Papular pruritic eruption • Fungal nail infections • Seborrhoeic dermatitis |
|
Clinical stage 3 |
|
• Unexplained severe weight loss (>10% of presumed or measured body weight) • Unexplained chronic diarrhoea for longer than 1 month • Unexplained persistent fever (intermittent or constant for longer than 1 month) • Persistent oral candidiasis • Oral hairy leukoplakia • Pulmonary TB • Severe bacterial infections (such as pneumonia, empyema, pyomyositis, bone or joint infection, meningitis, bacteraemia) • Acute necrotizing ulcerative stomatitis, gingivitis or periodontitis • Unexplained anaemia (<8 g/dl), neutropaenia (<0.5 × 109/l) and/or chronic thrombocytopaenia (<50 × 109/l) |
|
Clinical stage 4b |
|
• HIV wasting syndrome • Pneumocystis (jirovecii) pneumonia • Recurrent severe bacterial pneumonia • Chronic herpes simplex infection (orolabial, genital or anorectal of more than 1 month in duration or visceral at any site) • Oesophageal candidiasis (or candidiasis of trachea, bronchi or lungs) • Extrapulmonary TB • Kaposi sarcoma • Cytomegalovirus infection (retinitis or infection of other organs) • Central nervous system toxoplasmosis • HIV encephalopathy • Extrapulmonary cryptococcosis, including meningitis • Disseminated nontuberculous mycobacterial infection • Progressive multifocal leukoencephalopathy • Chronic cryptosporidiosis • Chronic isosporiasis • Disseminated mycosis (extrapulmonary histoplasmosis, coccidioidomycosis) • Lymphoma (cerebral or B-cell non-Hodgkin’s) • Symptomatic HIV-associated nephropathy or cardiomyopathy • Recurrent septicaemia (including non-typhoidal Salmonella) • Invasive cervical carcinoma • Atypical disseminated leishmaniasis |
a In the development of this table, adolescents were defined as 15 years or older.
b Some additional specific conditions can be included in regional classifications, such as penicilliosis in Asia, HIV-associated rectovaginal fistula in southern Africa and reactivation of trypanosomiasis in Latin America.