Introduction
It is imperative to re-think the way aerosol therapy is being practiced with a mindset shift from the rampant use of nebulizers to an alternative form of drug delivery. GINA and WHO Guidelines have recommended to avoid the use of nebulizers to reduce risk of transmitting viral infection. pMDI + Spacer is the first line of therapy for acute cases as per GINA, has been shown to be equivalent to nebulizer at a much lower dose, it is preferred by patients and requires minimal maintenance.
Acute Exacerbations of Asthma
Exacerbations represent an acute or sub-acute worsening in symptoms and lung function from the patient’s usual status, characterized by a progressive increase in symptoms of shortness of breath, cough, wheezing and chest tightness. The likelihood and frequency of exacerbations is reduced if the underlying condition is well managed, yet many patients still suffer acute exacerbations.
Exacerbations of asthma are unpleasant and frightening, and often lead to emergency visits to hospital, admission and in more severe cases, even death. Effective administration of inhaled drugs in high doses, along with supplementary oral medications, is the key to initial exacerbation of asthma, within primary health clinic and hospital settings.
The pMDI + Spacer approach has been studied and documented in various randomized controlled clinical trials and recognized in international guidelines (GINA/WHO). Using of a pressurized metered dose inhaler (pMDI) along with spacer has been found to be most effective way for administering these treatments.3 This approach is equally effective as nebulized therapy for administering bronchodilators in the management of acute asthma.
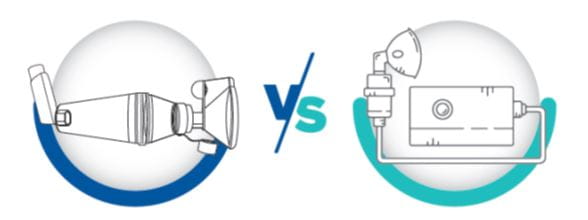
Comparing pMDI + Spacer vs Nebulizers in Acute Exacerbations of Asthma
Extensive research is available which suggests that, in situations where emergency administration of a bronchodilator is indicated for an acute exacerbation of asthma in both children and adults, the use of a pMDI with a spacer is as effective and safe as nebulized therapy, and may indeed reduce emergency room waiting times.4
Infants and young children lack the coordination required to trigger and simultaneously inhale a drug when using pMDIs, but the use of adjuncts such as spacers or face masks overcomes this difficulty.5
Nebulizers require a power supply, take more time, are not conveniently portable, are generally more expensive, require maintenance, and need more supervision. In contrast, spacers are easier to use, require less effort and time, do not require dose preparation or electricity for delivery, are portable, and require lower medication doses compared to nebulizers.4,6
When using a nebulizer, less than 10% of the aerosolized drug reaches the lungs, with large deposits remaining in the apparatus or on the face, and the remainder lost to the surroundings. In comparison, pMDIs + spacers cause no wastage of drug into surroundings and have a pulmonary deposition of 10% to 40%, depending upon the device type.5
Also, with the use of Nebulizers, there is a high chance of cross infections, as nebulizers can transmit respiratory pathogens. Nebulization requires regular cleaning and the cleaning is a detailed tedious process to avoid cross infections.5
GINA Recommendations for Adults and Adolescents with Acute Exacerbation1
The recommendations for management of exacerbations by Global Initiative for Asthma GINA 2020 guidelines are:
- For mild to moderate exacerbations, repeated administrations of inhaled SABA (upto 4-10 puffs) via pMDI + spacer every 20 minutes in the first hour, followed by every 3-4 hours if required.
- Delivery of a SABA via pMDI + Spacer leads to a similar improvement in lung function as delivery via nebulizer, in a cost-effective manner
GINA Recommendations for Children 5 Years and Younger with Acute Exacerbation1
- Inhaled therapy constitutes the cornerstone of asthma treatment in children 5 years and younger. A pMDI with a valved spacer (with or without a face mask, depending on the child’s age is the preferred delivery system)
- In the acute setting, initiate treatment with 2 puffs of inhaled SABA, given one puff at a time, via a pMDI + Spacer device, with or without a facemask. This may be repeated at 20 minutes intervals if no improvement in symptoms.
Implications in Current Scenario
- Use of nebulizers was found to be associated with a major outbreak of Severe Acute Respiratory Syndrome (SARS) in Hong Kong in 2003. Under prevailing situation which is gripping the entire world, it is imperative to restrict the use of nebulizers and make the switch to pMDI + Spacer.
- GINA guidelines1 emphasize to avoid the use of nebulizers wherever possible, to reduce the risk of transmission of infection to healthcare workers and other patients. Instead, GINA advises to use pMDI + Spacer (with or without face mask) to deliver SABA to adults and children for acute asthma.
Guidelines from WHO2
World Health Organization clearly states that airborne transmission may be possible in specific circumstances and settings in which procedures or support treatments that generate aerosols are performed. Airborne transmission refers to the presence of microbes within droplet nuclei, (particles <5μm); which can remain in the air for long periods of time and be transmitted to others over distances greater than 1 m.
Hence, WHO recommends to avoid use of nebulizers wherever possible and take sufficient airborne precautions for those exposed.
In conclusion, there is enough scientific evidence to encourage patients and health care professionals to embrace the pMDI + Spacer combination for treatment of asthma in the acute setting.
References
- GINA Guidelines 2020
- https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations, Last accessed on 3rd May 2020
- Lancet Respir Med 2019; May 7(5), 380-382
- ERJ Open Research 2018 4: 00065-2018; DOI: 10.1183/23120541.00065-2018
- Can Fam Physician. 2012; 58(5): 528–530
- Intern J Ped and Adol Med. 2014;1(1):26–30
Disclaimer: This scientific content are medically validated excerpts, strictly for the use of Registered Medical Practitioners, solely for knowledge upgradation and informational purpose. It is not intended towards any product promotion. Cipla makes every effort to present accurate and reliable information, but does not endorse, warrant, or assume any legal liability or responsibility for, the accuracy or completeness of any information provided. Cipla hereby disclaims all warranties regarding the contents, including without limitation all warranties of title, non-infringement, merchantability, and fitness for a particular purpose. Any change in the treatment regime is totally in discretion of the physician/ doctor. No part of this may be reproduced, transmitted or stored in any form or by any means either mechanically or electronically.

.svg?iar=0&updated=20230109065058&hash=B8F025B8AA9A24E727DBB30EAED272C8)