Part I: Health Professional Information
Abridged Prescribing Information1
Carvedilol 3.125mg/6.25mg/12.5mg and Ivabradine 5mg Tablets
CARLOC-I
Composition
Carvedilol IP………………………………………………… 3.125 mg
Ivabradine hydrochloride equivalent to Ivabradine………..... 5mg
Carvedilol IP………………………………………………… 6.25 mg
Ivabradine hydrochloride equivalent to Ivabradine……......... 5mg
Carvedilol IP…………………………………………………12.5 mg
Ivabradine hydrochloride equivalent to Ivabradine……….... 5mg
Dosage Form and Strength
Carvedilol 3.125mg/6.25mg/12.5mg and Ivabradine 5mg Tablets
Therapeutic Indications
CARLOC-I is indicated for as substituted therapy in adult patients with normal sinus rhythm already controlled by Ivabradine and Carvedilol taken concomitantly at the same doses level for: the symptomatic treatment of chronic stable angina pectoris in coronary artery disease patients, the treatment of chronic heart failure (II-IV NYHA-Class) with systolic dysfunction
Dosage and Administration
The recommended dose of the CARLOC-I is one tablet twice-daily, once in the morning and once in the evening. CARLOC-I should only be used in patients controlled on stable doses of the individual components given concurrently when carvedilol and ivabradine are at the optimal dose. The CARLOC-I is not suitable for initiation therapy. If a change of posology is required, titration should be done with the individual components carvedilol and ivabradine, ensuring the patient is maintained at an optimal dose of carvedilol and ivabradine. It is recommended that the decision to titrate treatment takes place with the availability of serial heart rate measurements, ECG or ambulatory 24-hour monitoring. If during treatment, heart rate decreases below 50 beats per minute at rest or the patient experiences symptoms related to bradycardia such as dizziness, fatigue or hypotension, down titration should be done with the individual components carvedilol and ivabradine, ensuring the patient is maintained at an optimal dose of carvedilol and ivabradine. After dose reduction, heart rate should be monitored. Treatment must be discontinued if heart rate remains below 50 bpm or symptoms of bradycardia persist despite dose reduction.
Contraindications
CARLOC-I is contraindicated in patients with — Hypersensitivity to the active substances or to any other beta-blockers, Severe hepatic impairment, Acute or unstable/decompensated heart failure, Unstable Angina, Prinzmetal's angina, AV-block of 2nd and 3rd degree, Sick sinus syndrome (including sino-atrial block), Symptomatic or severe bradycardia (<50 bpm), Acute myocardial infarction, Cardiogenic Shock, Pacemaker dependent (heart rate imposed exclusively by the pacemaker), Severe peripheral vascular disease (e.g. Raynaud's phenomenon), Severe hypotension (systolic arterial blood pressure <90 mmHg, diastolic arterial blood pressure <50 mmHg), Chronic obstructive pulmonary disease associated with bronchial obstruction, History of bronchospasm or asthma, Metabolic acidosis, Untreated phaeochromocytoma, Combination with verapamil or diltiazem which are moderate CYP3A4 inhibitors with heart rate reducing properties, Combination with strong cytochrome P450 3A4 inhibitors such as azole antifungals (ketoconazole, itraconazole), macrolide antibiotics (clarithromycin,, erythromycin per os, josamycin, telithromycin), HIV protease inhibitors (nelfinavir, ritonavir) and nefazodone, Pregnancy, lactation and women of child-bearing potential not using appropriate contraceptive measures.
Warnings and Precautions
- Special warnings
Lack of benefit on clinical outcomes in patients with symptomatic chronic stable angina pectoris.
CARLOC-I is indicated only for symptomatic treatment of chronic stable angina pectoris because ivabradine has no benefits on cardiovascular outcomes (e.g. myocardial infarction or cardiovascular death).
- Measurement of Heart Rate
Given that the heart rate may fluctuate considerably over time, serial heart rate measurements, ECG or ambulatory 24-hour monitoring should be considered when determining resting heart rate in patients on treatment with ivabradine when titration is considered. This also applies to patients with a low heart rate, in particular when heart rate decreases below 50 bpm, or after dose reduction.
- Cardiac Arrhythmias
Ivabradine is not effective in the treatment or prevention of cardiac arrhythmias and likely loses its efficacy when a tachyarrhythmia occurs (e.g. ventricular or supraventricular tachycardia). CARLOC-I is therefore not recommended in patients with atrial fibrillation or other cardiac arrhythmias that interfere with sinus node function.
In patients treated with ivabradine, the risk of developing atrial fibrillation is increased. Atrial fibrillation has been more common in patients using concomitantly amiodarone or potent class I anti-arrhythmics. It is recommended to regularly clinically monitor ivabradine treated patients for the occurrence of atrial fibrillation (sustained or paroxysmal), which should also include ECG monitoring if clinically indicated (e.g. in case of exacerbated angina, palpitations, irregular pulse).
Patients should be informed of signs and symptoms of atrial fibrillation and be advised to contact their physician if these occur. If atrial fibrillation develops during treatment, the balance of benefits and risks of continued treatment of CARLOC-I should be carefully reconsidered. Chronic heart failure patients with intraventricular conduction defects (bundle branch block left, bundle branch block right) and ventricular dyssynchrony should be monitored closely.
- Use in Patients with a Low Heart Rate
CARLOC-I must not be initiated in patients with a pre-treatment resting heart rate below 50 beats per minute. If, during treatment with CARLOC-I, resting heart rate decreases persistently below 50 bpm or the patient experiences symptoms related to bradycardia such as dizziness, fatigue or hypotension, down titration should be done with the individual components ensuring the patient is maintained at an optimal dose of carvedilol and ivabradine or treatment discontinued.
- Combination with Calcium Channel Blockers
Concomitant use of CARLOC-I with heart rate reducing calcium channel blockers such as verapamil or diltiazem is contraindicated. No safety issue has been raised on the combination of ivabradine with nitrates and dihydropyridine calcium channel blockers such as amlodipine. Additional efficacy of ivabradine in combination with dihydropyridine calcium channel blockers has not been established.
- Chronic Heart Failure
Heart failure must be stable before considering treatment with the CARLOC-I. The FDC of carvedilol and ivabradine is not recommended in heart failure patients with NYHA functional classification IV due to limited amount of data with ivabradine in this population.
CARLOC-I should be used with caution in combination with digitalis glycosides since these products, as well as carvedilol, may slow the AV conduction.
- Stroke
The use of the FDC of carvedilol and ivabradine is not recommended immediately after a stroke since no data with ivabradine is available in these situations.
- Visual Function
Ivabradine influences retinal function. There is no evidence of a toxic effect of long-term ivabradine treatment on the retina. Cessation of treatment should be considered if any unexpected deterioration in visual function occurs. Caution should be exercised in patients with retinitis pigmentosa.
- Precautions for use
- Stopping Treatment
Ivabradine intake can be interrupted if necessary, however an abrupt cessation of therapy with a beta-blocker should be avoided, especially in patients with ischaemic heart disease. The cessation of the FDC of carvedilol and ivabradine therapy should immediately be followed by the intake of carvedilol individual tablet ensuring the patient is maintained at an optimal dose of carvedilol. Posology of individual carvedilol should be decreased gradually.
- Renal Function in Congestive Heart Failure
Reversible deterioration of renal function has been observed with carvedilol therapy in chronic heart failure patients with low arterial blood pressure (SBP <100 mmHg), ischaemic heart disease and diffuse vascular disease, and/or underlying renal insufficiency.
- Patients with Hypotension
Limited data are available in patients with mild to moderate hypotension, and ivabradine should therefore be used with caution in these patients. CARLOC-I is contraindicated in patients with severe hypotension (systolic arterial blood pressure <90 mmHg, diastolic arterial blood pressure <50 mmHg).
- Atrial Fibrillation - Cardiac Arrhythmias
The use of CARLOC-I in patients with congenital QT syndrome or treated with QT prolonging medicinal products should be avoided. If the combination appears necessary, close cardiac monitoring is needed. Heart rate reduction, as caused by ivabradine, may exacerbate QT prolongation, which may give rise to severe arrhythmias, in particular Torsade de pointes.
- Hypertensive Patients Requiring Blood Pressure Treatment Modifications
When treatment modifications are made in chronic heart failure patients treated with ivabradine blood pressure should be monitored at an appropriate interval.
- Diabetic Patients
Close monitoring of diabetic patients receiving the FDC of carvedilol and ivabradine is required by means of regular blood glucose measurements and adjustment of anti-diabetic medication as necessary.
- Peripheral Vascular Disease
CARLOC-I should be used with caution in patients with peripheral vascular diseases, as beta-blockers may precipitate or aggravate symptoms of the disease.
- Anaesthesia and Major Surgery
Beta-blockers reduce the risk of arrhythmias under anaesthesia, but the risk of hypotension may be increased. Caution should therefore be applied when using certain anaesthetics due to the negative synergic, inotropic effects of carvedilol and anaesthetic products.
- Thyrotoxicosis/Hyperthyroidism
Beta-blockers, such as carvedilol, may mask the signs of hyperthyroidism and the symptoms of thyrotoxicosis.
- Contact Lenses
Patients who wear contact lenses and are treated with the FDC of carvedilol and ivabradine should be advised of the possible reduction of lachrymal secretion due to the carvedilol component.
- Hypersensitivity
The FDC of carvedilol and ivabradine should be used with caution in patients with a history of serious hypersensitivity reactions and in those undergoing desensitisation therapy as beta-blockers, such as carvedilol, may increase both the sensitivity towards allergens and the seriousness of anaphylactic reactions.
- Psoriasis
In patients with a personal or family history of psoriasis associated with beta-blocker therapy, the FDC of carvedilol and ivabradine should only be prescribed after a careful weighing of risks and benefits as beta-blockers may worsen the skin reactions.
- Phaeochromocytoma
Caution should be considered when in the administration of the FDC of carvedilol and ivabradine to patients suspected of having phaeochromocytoma.
Further Precautions
Due to insufficient clinical data, carvedilol should not be administered to patients with labile or secondary hypertension, orthostatic hypotension, acute myocarditis, a haemodynamically relevant stenosis of the heart valves or ventricular outflow tract, end-stage peripheral arterial disease or who are concomitantly receiving an α1-receptor antagonist or α2-receptor agonist.
Pharmacodynamic Properties
Carvedilol
In hypertensive patients, a reduction in blood pressure is not associated with a concomitant increase in peripheral resistance, as observed with pure beta-blockers. The heart rate is slightly decreased. Stroke volume remains unchanged. Renal blood flow and renal function remain normal, as does peripheral blood flow. Therefore, cold extremities, which often occur with beta-blockers, are rarely seen. In hypertensive patients, carvedilol increases the plasma norepinephrine concentration.
In prolonged treatment of patients with angina pectoris, carvedilol has been seen to have an anti-ischaemic effect and to alleviate pain. Haemodynamic studies have demonstrated that carvedilol reduces ventricular pre- and after-load.
In patients with left ventricular dysfunction or congestive heart failure, carvedilol has a favourable effect on haemodynamics and the left ventricular ejection fraction and its dimensions. Carvedilol reduces mortality and the need for cardiovascular hospitalisation in patients with heart failure.
Carvedilol has no negative effect on the serum lipid profile or electrolytes. The ratio of high-density lipoproteins and low-density lipoproteins remains normal.
Ivabradine
The main pharmacodynamic property of ivabradine in humans is a specific dose dependent reduction in heart rate. Analysis of heart rate reduction with doses up to 20 mg twice-daily indicates a trend towards a plateau effect, which is consistent with a reduced risk of severe bradycardia below 40 bpm.
At usual recommended doses, heart rate reduction is approximately 10 bpm at rest and during exercise. This leads to a reduction in cardiac workload and myocardial oxygen consumption. Ivabradine does not influence intracardiac conduction, contractility (no negative inotropic effect) or ventricular repolarisation:
- in clinical electrophysiology studies, ivabradine had no effect on atrioventricular or intraventricular conduction times or corrected QT intervals;
- in patients with left ventricular dysfunction (left ventricular ejection fraction (LVEF) between 30 and 45%), ivabradine did not have any deleterious influence on LVEF.
Pharmacokinetic Properties
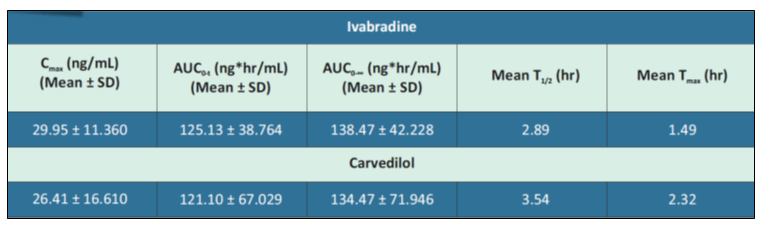
A bioequivalence (BE) study was conducted to compare the single dose oral bioequivalence of this FDC (Ivabradine 5 mg and Carvedilol 12.5 mg) versus the same composition of the innovator FDC in healthy, adult, male human subjects under fed condition. The study showed that this FDC formulation when compared to the same composition of the innovator FDC met the relative bioavailability criteria as the statistical outcome of 90% Confidence Intervals of the ratios of log-transformed primary pharmacokinetic parameters of Cmax, AUC0-t and AUC0-∞ for Carvedilol & Ivabradine were in the acceptable range of 80% to 125%. Hence, as per this study it is concluded that this FDC (Ivabradine 5 mg and Carvedilol 12.5 mg) is bioequivalent to the same composition of the innovator FDC when administered as a single oral dose in healthy, adult, male human subjects under fed condition. Also, the FDC of Carvedilol and Ivabradine was well-tolerated as a single dose in all subjects. There were no adverse events during the entire study period.
Mean pharmacokinetic parameters of Ivabradine and Carvedilol in healthy male subjects after single dose oral administration of the FDC of Ivabradine 5 mg and Carvedilol 12.5 mg prolonged release tablets under fed condition are shown in Table 1.
The detailed pharmacokinetics of individual Ivabradine and Carvedilol molecules is as given below
Carvedilol
Absorption
The absolute bioavailability of carvedilol administered orally is approximately 25%. Maximum plasma concentration is achieved approximately 1 hour after administration. There is a linear relationship between dose and plasma concentrations. In patients with a slow debrisoquine hydroxylation, carvedilol's plasma concentration increased by a factor of 2 to 3, compared with rapid metabolisers of debrisoquine. Food intake does not affect bioavailability, although it takes longer to reach maximum plasma concentration.
Distribution
Carvedilol is highly lipophilic. Plasma protein binding is about 98 to 99%. The distribution volume is around 2 L/kg. The first-pass effect after oral administration is around 60 - 75%.
Biotransformation
Carvedilol is extensively metabolised to various metabolites which are excreted primarily via bile. The first pass metabolism after oral administration is about 60-75%. The enterohepatic circulation of the parent substance has been demonstrated in animals. Carvedilol is metabolised in the liver, mainly through oxidation of the aromatic ring and glucuronidation. Demethylation and hydroxylation at the phenol ring produce three active metabolites with beta-blocking activity. These three active metabolites have a weak vasodilating effect, compared with carvedilol.
According to preclinical studies, the beta-blocking activity of the metabolite 4-hydroxyphenol is approximately 13 times higher than that of carvedilol. However, the metabolite concentrations in humans are about 10 times lower than that of carvedilol. Two of the carbazole-hydroxy metabolites of carvedilol are extremely potent antioxidants, making them 30 - 80 times stronger than carvedilol.
The oxidative metabolism of carvedilol is stereoselective. R-enantiomer is primarily metabolised by CYP2D6 and CYP1A2, while S-enantiomer is primary metabolised by CYP2C9 and to a lesser extent by CYP2D6. Other CYP450 isoenzymes participating in carvedilol metabolism include CYP3A4, CYP2E1 and CYP2C19. Maximum plasma concentration of R-carvedilol in plasma is approximately twice the concentration of S-carvedilol. R-enantiomer is metabolised mainly via hydroxylation. In the slow metabolisers of CYP2D6, an increase of carvedilol concentration in plasma may occur, mainly of the R-enantiomer, leading to the increase of the alpha-blocking activity.
Elimination
The average half-life of elimination of carvedilol varies between 6 and 10 hours. The plasma clearance is approximately 590 mL/min. Elimination is mainly via bile. Excretion is mainly via faeces. A minor part is eliminated renally in the form of metabolites.
Special Populations
Elderly: The pharmacokinetics of carvedilol is dependent on age. Plasma carvedilol levels are around 50% higher in the elderly than in young people.
Hepatic Impairment: In a study involving patients with liver cirrhosis, the bioavailability of carvedilol was four times higher and the maximum plasma concentration five times higher and the distribution volume three times higher than in healthy subjects.
Renal Impairment: In some hypertensive patients with moderate (creatinine clearance 20-30 mL/min) or severe (creatinine clearance <20 mL/min) renal impairment, an increase in plasma carvedilol concentrations of approximately 40-55% was seen compared to patients with normal renal function. However, there was a large variation in the results.
Ivabradine
Under physiological conditions, ivabradine is rapidly released from tablets and is highly water-soluble (>10 mg/mL). Ivabradine is the S-enantiomer with no bioconversion demonstrated in vivo. The N-desmethylated derivative of ivabradine has been identified as the main active metabolite in humans.
Absorption and Bioavailability
Ivabradine is rapidly and almost completely absorbed after oral administration with a peak plasma level reached in about 1 hour under fasting condition. The absolute bioavailability of the film-coated tablets is around 40%, due to first-pass effect in the gut and liver. Food delayed absorption by approximately 1 hour, and increased plasma exposure by 20 to 30%. The intake of the tablet during meals is recommended in order to decrease intra-individual variability in exposure.
Distribution
Ivabradine is approximately 70% plasma protein bound and the volume of distribution at steady state is close to 100 L in patients. The maximum plasma concentration following chronic administration at the recommended dose of 5 mg twice daily is 22 ng/mL (CV=29%). The average plasma concentration is 10 ng/mL (CV=38%) at steady state.
Biotransformation
Ivabradine is extensively metabolised by the liver and the gut by oxidation through cytochrome P450 3A4 (CYP3A4) only. The major active metabolite is the N-desmethylated derivative (S 18982) with an exposure about 40% of that of the parent compound. The metabolism of this active metabolite also involves CYP3A4. Ivabradine has low affinity for CYP3A4, shows no clinically relevant CYP3A4 induction or inhibition and is therefore unlikely to modify CYP3A4 substrate metabolism or plasma concentrations. Inversely, potent inhibitors and inducers may substantially affect ivabradine plasma concentrations.
Elimination
Ivabradine is eliminated with a main half-life of 2 hours (70-75% of the AUC) in plasma and an effective half-life of 11 hours. The total clearance is about 400 mL/min and the renal clearance is about 70 mL/min. Excretion of metabolites occurs to a similar extent via faeces and urine. About 4% of an oral dose is excreted unchanged in urine.
Linearity/non linearity
The kinetics of ivabradine is linear over an oral dose range of 0.5 – 24 mg.
Special Populations
Elderly: no pharmacokinetic differences (AUC and Cmax) have been observed between elderly (≥ 65 years) or very elderly patients (≥75 years) and the overall population.
Renal Impairment: the impact of renal impairment (creatinine clearance from 15 to 60 mL/min) on ivabradine pharmacokinetic is minimal, in relation with the low contribution of renal clearance (about 20 %) to total elimination for both ivabradine and its main metabolite S 18982.
Hepatic Impairment: in patients with mild hepatic impairment (Child Pugh score up to 7) unbound AUC of ivabradine and the main active metabolite were about 20% higher than in subjects with normal hepatic function. Data are insufficient to draw conclusions in patients with moderate hepatic impairment. No data are available in patients with severe hepatic impairment.
Pharmacokinetic/Pharmacodynamic (PK/PD) Relationship
PK/PD relationship analysis has shown that heart rate decreases almost linearly with increasing ivabradine and S 18982 plasma concentrations for doses of up to 15-20 mg twice daily. At higher doses, the decrease in heart rate is no longer proportional to ivabradine plasma concentrations and tends to reach a plateau. High exposures to ivabradine that may occur when ivabradine is given in combination with strong CYP3A4 inhibitors may result in an excessive decrease in heart rate although this risk is reduced with moderate CYP3A4 inhibitors.
Interactions
Specific drug-drug interaction studies have shown no clinically significant effect of the following medicinal products on pharmacokinetics and pharmacodynamics of ivabradine: proton pump inhibitors (omeprazole, lansoprazole), sildenafil, HMG CoA reductase inhibitors (simvastatin), dihydropyridine calcium channel blockers (amlodipine, lacidipine), digoxin and warfarin. In addition, there was no clinically significant effect of ivabradine on the pharmacokinetics of simvastatin, amlodipine, lacidipine, on the pharmacokinetics and pharmacodynamics of digoxin, warfarin and on the pharmacodynamics of aspirin.
Use in Special Populations
- Patients with Renal Impairment
Ivabradine
No dosage adjustment is required for patients with creatinine clearance 15 to 60 mL/min. No data are available for patients with creatinine clearance below 15 mL/min.
Carvedilol
Although carvedilol is metabolized primarily by the liver, plasma concentrations of carvedilol have been reported to be increased in patients with renal impairment. Based on mean AUC data, approximately 40% to 50% higher plasma concentrations of carvedilol were observed in subjects with hypertension and moderate to severe renal impairment compared with a control group of subjects with hypertension and normal renal function. However, the ranges of AUC values were similar for both groups. Changes in mean peak plasma levels were less pronounced, approximately 12% to 26% higher in subjects with impaired renal function.
Consistent with its high degree of plasma protein-binding, carvedilol does not appear to be cleared significantly by hemodialysis.
- Patients with Hepatic Impairment
Ivabradine
No dose adjustment is required in patients with mild or moderate hepatic impairment. Ivabradine is contraindicated in patients with severe hepatic impairment (Child-Pugh C) as it has not been studied in this population and an increase in systemic exposure is anticipated.
Carvedilol
Compared with healthy subjects, patients with severe liver impairment (cirrhosis) exhibit a 4-to 7-fold increase in carvedilol levels. Carvedilol is contraindicated in patients with severe liver impairment.
- Women of Child-bearing Potential
Women of child-bearing potential should use appropriate contraceptive measures during treatment.
- Pregnant Women
Based on existing data with the individual components, the use of the FDC of carvedilol and ivabradine is contra-indicated during pregnancy.
Lactating Women
The FDC of carvedilol and ivabradine is contra-indicated during breast-feeding.
- Geriatric Patients
Carvedilol
Plasma levels of carvedilol average about 50% higher in the elderly compared with young subjects. Of the 765 subjects with heart failure randomized to carvedilol in U.S. clinical trials, 31% (235) were aged 65 years or older, and 7.3% (56) were aged 75 years or older. Of the 1,156 subjects randomized to carvedilol in a long-term, placebo-controlled trial in severe heart failure, 47% (547) were aged 65 years or older, and 15% (174) were aged 75 years or older. Of 3,025 subjects receiving carvedilol in heart failure trials worldwide, 42% were aged 65 years or older. Of the 975 subjects with myocardial infarction randomized to carvedilol in the CAPRICORN trial, 48% (468) were aged 65 years or older, and 11% (111) were aged 75 years or older. Of the 2,065 hypertensive subjects in U.S. clinical trials of efficacy or safety who were treated with carvedilol, 21% (436) were aged 65 years or older. Of 3,722 subjects receiving carvedilol in hypertension clinical trials conducted worldwide, 24% were aged 65 years or older. With the exception of dizziness in hypertensive subjects (incidence 8.8% in the elderly versus 6% in younger subjects), no overall differences in the safety or effectiveness were observed between the older subjects and younger subjects in each of these populations. Similarly, other reported clinical experience has not identified differences in responses between the elderly and younger subjects, but greater sensitivity of some older individuals cannot be ruled out.
Ivabradine
No pharmacokinetic differences have been observed in elderly (≥ 65 years) or very elderly (≥ 75 years) patients compared to the overall population. However, ivabradine has only been studied in a limited number of patients ≥ 75 years of age.
Pharmaceutical Particulars
Incompatibilities
Not Known
Shelf-life
24 Months
Packaging Information
Pack of 10 Tablets
Storage and Handing Instructions
Store at a temperature not exceeding 30°C
Part II: Scientific Information
Description
CARLOC-I is a fixed dose combination of Carvedilol and Ivabradine.
Carvedilol
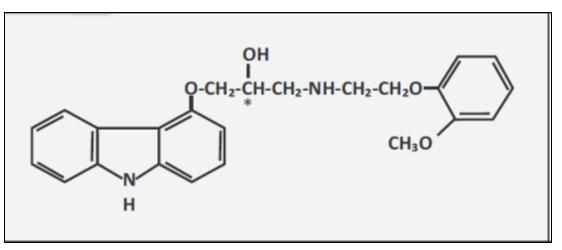
Carvedilol is a nonselective β-adrenergic blocking agent with α1-blocking activity. It is a racemic mixture with the following structure (see Fig. 1).2
Chemical Name: (±)-1 (Carbazol-4-yloxy)-3-[[2-(o-methoxyphenoxy)ethyl]amino]-2-propanol
Molecular Formula: C24H26N2O4
Molecular Weight: 406.5
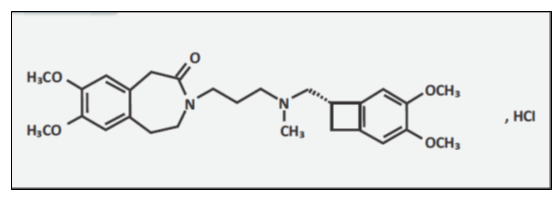
Ivabradine
Ivabradine is a hyperpolarization-activated cyclic nucleotide-gated channel blocker that reduces the spontaneous pacemaker activity of the cardiac sinus node by selectively inhibiting the If current. This results in heart rate reduction with no effect on ventricular repolarization and no effects on myocardial contractility.3
Chemical Name: 3-(3-{[((7S)-3,4-Dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-yl)methyl] methyl amino} propyl)-1,3,4,5-tetrahydro-7,8-dimethoxy-2H-3-benzazepin-2-one, hydrochloride. The chemical structure of ivabradine is shown in Fig. 2.
Molecular Formula: C27H36N2O5· HCl
Molecular Weight: (free base + HCl) is 505.1 (468.6 + 36.5)
Mechanism of Action
Carvedilol
Carvedilol is a non-selective β-blocker with α1-adrenergic receptor antagonist properties. It is a non-selective, cardiac β-blocker with peripheral vasodilating effects. Secondary to its unique action, carvedilol maintains cardiac output by decreasing afterload in conjunction with a cardiac β-blockade and has a lesser effect on heart rate than pure selective β-blockers. Other benefits include antioxidant effects, reduction in neutrophil infiltration and apoptosis inhibition. As it prevents the formation of oxidized low-density lipoproteins and inhibit vascular smooth muscle cell proliferation and migration, it shows a great promise in the treatment of atherosclerotic disease formation and progression. Carvedilol decreases blood pressure mainly by decreasing arterial vascular resistance through its α1-blocking properties, causing a reduction in afterload. It is highly useful in the management of hypertension in patients with renal impairment, where clinicians should avoid diuretics and angiotensin-converting enzyme inhibitors (ACEI). Plasma renin activity is reduced and fluid retention is rare. Carvedilol has no intrinsic sympathomimetic activity. Like propranolol, it has membrane-stabilising properties. Carvedilol is a racemate of two stereoisomers. Both enantiomers were found to have alpha-adrenergic blocking characteristics in animal experiments. Non-selective beta1- and beta2- adrenoceptor blockade is attributed mainly to the S(-) enantiomer. The antioxidant properties of carvedilol and its metabolites have been demonstrated in in vitro and in vivo animal studies and in vitro in a number of human cell types.1,4
Ivabradine
Ivabradine lowers heart rate by selectively inhibiting If channels (funny channels) in the heart in a concentration-dependent manner without affecting any other cardiac ionic channels (including calcium or potassium). The cardiac effects are more pronounced in the sinoatrial (SA) node, but prolongation of the AH interval has occurred as has PR interval prolongation. There was no effect on ventricular repolarization and myocardial contractility. Ivabradine can also inhibit retinal current Ih that resembles cardiac If. It participates in the temporal resolution of the visual system by curtailing retinal responses to bright light stimuli. Under triggering circumstances (e.g., rapid changes in luminosity), partial inhibition of Ih by Corlanor may underlie the luminous phenomena experienced by patients.1,3,5
Clinical Trials on Carvedilol and Ivabradine Combination
The efficacy of ivabradine combined with a β-blocker was demonstrated during the clinical development of ivabradine. The concomitant use of β-blockers and ivabradine is efficacious in the treatment of CHF. The therapeutic guidelines also recommend ivabradine/β-blocker for the management of CHF. The below mentioned clinical trials presents an overview of the use of β-blockers/ivabradine and of carvedilol/ivabradine in reducing the heart rate.6
Efficacy of Carvedilol/Ivabradine Combination on Heart Rate, QOL, Morbidity and Mortality in Patients with Stable Ischemic Heart Failure
Increased heart rate >60 beats/min in heart failure (HF) patients is a strong predictor of death and hospitalisation. This led to hypothesis that lowering the heart rate with ivabradine might be beneficial for cardiac function and clinical outcomes in HF patients. A study conducted in stable ischemic heart failure (IHF) patients assessed the efficacy of carvedilol and carvedilol/ivabradine combination. Patients were randomised to receive either carvedilol up to 25 mg BID (group I) and carvedilol/ivabradine up to 25/7.5 mg BID (group II). The follow-up period was 6 months. The average daily dose of carvedilol was 16.2 mg and 11.4 mg in group I and in group II respectively.7
The clinical findings of the study:
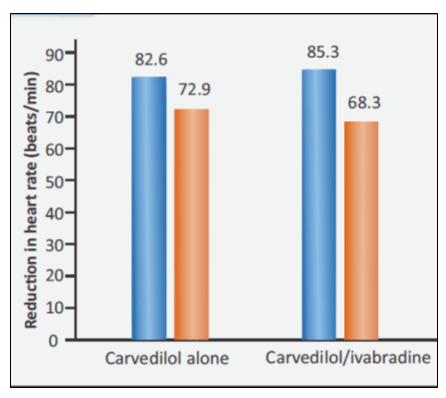
There was significant decrease in resting heart rate in both the groups. But the decrease in heart rate was higher in the combination group (see Fig. 3).
- GROUP I – 82.6 to 72.9 beats/min
- GROUP II - 85.3 to 68.5 beats/min
- Left ventricular ejection fraction (LVEF) was significantly increased in group II by 4.6 (5.9)% (p=0.01), whereas no significant change was observed in group I.
- Health-related quality of life (QOL) was significantly improved and hospital admissions due to worsening heart failure were also reduced with the carvedilol/ivabradine combination.
Ivabradine combined with carvedilol is associated with better heart rate reduction, improvement of quality of life, and reduced rehospitalization in stable IHF patients with heart rate >70 beats/min.
Significant Reduction in HR with Carvedilol/Ivabradine Lead to Reduced Cardiovascular Deaths or Hospitalisation
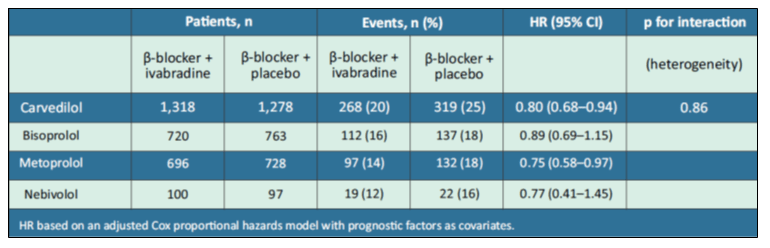
Another study explored the β-blockers prescription with ivabradine in patients with systolic HF, focusing on the most frequently coprescribed β-blocker, carvedilol. The authors analysed outcomes in SHIFT patients with systolic HF who were prescribed β-blockers (carvedilol, bisoprolol, metoprolol, or nebivolol) with ivabradine or placebo. Patients analysed were 2596 in carvedilol, 1483 in bisoprolol, 1424 in metoprolol and 197 in nebivolol. Treatment duration was 19 months.8
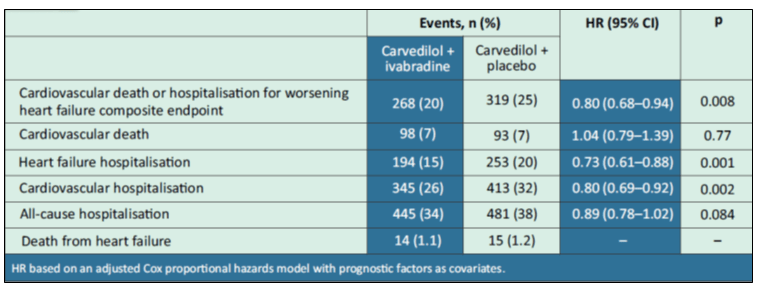
The primary endpoint is a composite of cardiovascular death and hospitalisation for worsening HF. In patients receiving carvedilol, the secondary endpoints were the individual components of the primary endpoint, heart failure death, cardiovascular hospitalisation, all-cause hospitalisation and the safety data.
Clinical Findings of the study were:
- There was no difference in the effect of ivabradine on the primary composite endpoint of cardiovascular death or heart failure hospitalisation between the various β-blockers [hazard ratios (HR) for risk reduction, 0.75–0.89; p for interaction = 0.86; see Table 2].
- Carvedilol/ivabradine showed lower rates of primary composite endpoint (HR 0.80, 95% CI: 0.68–0.94), heart failure hospitalisation (HR 0.73, 95% CI: 0.61–0.88), and cardiovascular hospitalisation (HR 0.80, 95% CI: 0.69–0.92) compared to carvedilol with placebo (see Table 3).
- The dosage of carvedilol had no detectable effect and there were no unexpected safety issues.
- After 28 days and after 1 year, heart rate were 63.8±10.7 and 64.3±11.1 bpm, respectively, for patients receiving carvedilol/ivabradine vs. 75.2±11.8 and 74.4±12.6 bpm, respectively, for those receiving carvedilol/placebo.
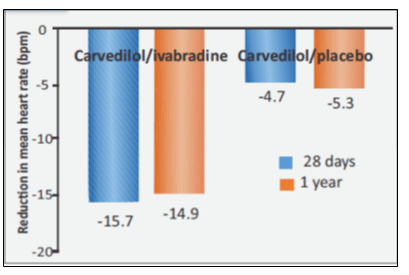
- Reduction in mean heart rate was greater with carvedilol/ivabradine compared to carvedilol/placebo (see Fig. 4).
At 28 days and at 1 year heart rate reduction in
- Carvedilol/ivabradine was –15.7±10.5 and –14.9±11.7 bpm, respectively
- Carvedilol/placebo was –4.7 ± 10.2 and –5.3 ± 12.1 bpm, respectively
- All the carvedilol dosage subgroups, there were fewer cardiovascular deaths or hospitalisation for worsening HF events with carvedilol/ivabradine than carvedilol/placebo [carvedilol 6.25 mg/day, 67 (29.1%) vs. 69 (36.9%) events, respectively; carvedilol 12.5 mg/day, 67 (22.3%) vs. 85 (26.2%) events; carvedilol 25 mg/day, 63 (20.4%) vs. 57 (20.5%) events, and carvedilol 50 mg/day, 48 (14.2%) vs. 65 (19.2%) events].
- Carvedilol/ivabradine was well-tolerated and there were no unexpected safety issues.
Prescription of β-blocker/ivabradine is associated with improved cardiovascular outcomes. In patients on carvedilol/ivabradine combination improved cardiovascular outcomes and was safe in patients with systolic HF in sinus rhythm with a resting heart rate ≥70 bpm. There was significant reduction in heart rate with carvedilol/ivabradine that led to fewer cardiovascular deaths or hospitalisation for worsening HF events.
Early Treatment with Ivabradine/β-Blockers vs. β-Blockers alone in Patients Hospitalised with HF and Reduced LVEF: ETHIC-AHF Trial
A comparative, randomised study analysed the efficacy of early coadministration of ivabradine and beta-blockers (carvedilol or bisoprolol; intervention group) vs. beta-blockers alone (control group) in patients hospitalised with heart failure and reduced left ventricular ejection fraction (HFrEF). A total of 71 patients with acute HF with LVEF <40% sinus rhythm, and heart rate >70 bpm were treated within 24 hours after hospital admission. Patients were randomised to either intervention group (33 patients) and control group (38 patients).9
Clinical Findings of the Study were:
- There were no differences in the baseline characteristics or standard treatment at discharge.
- At admission the heart rate was 87.3±10.6 bpm in the intervention group and 88.4±11.2 bpm in the control group.
- There was significant decrease in the heart rate at 28 days and at four months with the ivabradine/β-blockers
group compared to β-blockers alone.
- At 28 days, heart rate in ivabradine/β-blockers group vs. β-blockers alone was 64.3±7.5 vs. 70.3±9.3 bpm, p=0.01.
- At four months, heart rate in ivabradine/β-blockers group vs. β-blockers alone was 60.6±7.5 vs. 67.8±8 bpm, p=0.004.
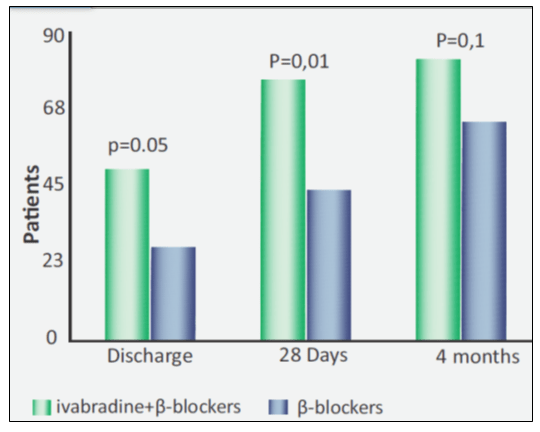
- The percentage of patients with target heart rate values (HR <70 bpm) was significantly higher in the ivabradine/β-blockers group compared to β-blockers alone at discharge and at 28 days after discharge (see Fig. 5).
- There were statistically significant differences between the two groups with respect to the ejection fraction at 4 months of follow-up (44.8±14.4% in the intervention group vs. 38.1±6.1% in the control group, p=0.039), as well as brain natriuretic peptide levels at 4 months of follow-up (259±78 vs. 554±192 pg/ml, p=0.02).
- Significant differences were found with respect to the EF and brain natriuretic peptide levels at four months.
- No differences in clinical events (rehospitalisation/death) were reported at four months.
- There were no severe side-effects with respect to the early administration of ivabradine were observed.
The early coadministration of ivabradine and β-blockers (carvedilol or bisoprolol) combination during hospital admission for acute HFrEF is feasible and safe. Ivabradine and β-blocker combination produces a significant decrease in heart rate at 28 days and at 4 months after hospital discharge. It also seemed to improve systolic function and functional and clinical parameters of HF patients at short-term.
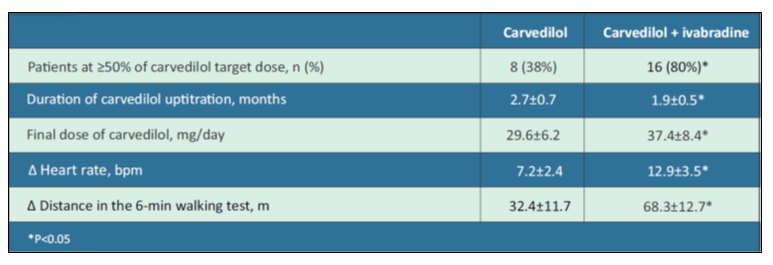
Addition of Ivabradine to Carvedilol reduces Duration of Carvedilol Uptitration and improves Exercise Capacity in Patients with CHF
Ivabradine improves prognosis in patients with CHF and is recommended by ESC Guidelines for symptomatic CHF patients with elevated heart rate on evidence-based or maximum tolerated doses of β-blocker and RAAS antagonists. However, many patients tolerate β-blockers poorly and few reach target dose. Based on this background, a study compared the uptitration of carvedilol alone or with ivabradine in CHF patients.10
A total of 41 patients in sinus rhythm with previous MI, CHF (NYHA class II-III), and heart rate ≥70 bpm were included. Patients included were not taken β-blockers for ≥2 months and were ivabradine-naive. They were randomised to carvedilol (21 patients) and carvedilol/ivabradine combination (20 patients). Carvedilol 3.125 mg BID was initiated on top of standard therapy, and doubled every 2 weeks until 25 mg BID or maximum tolerated dose was reached. Ivabradine 5 mg BID was prescribed 1-2 days after carvedilol initiation, and uptitrated to 7.5 mg BID 1 month later if heart rate ≥70 bpm. The final visit was at 3 months.
Clinical Findings of the Study were:
- The baseline heart rate was 83.8±11.6 bpm and baseline systolic blood pressure was 133.3±13.8 mm Hg.
- Table 4 depicts the study results at 3 months of carvedilol vs. carvedilol/ivabradine in CHF patients.
- The most common reasons for not reaching β-blockers target dose were hypotension, dizziness, and worsening CHF.
The addition of ivabradine to carvedilol in patients with CHF showed shorter β-blocker uptitration period, higher final β-blockers dose, greater heart rate reduction, and better exercise capacity.
Part III: Patient Counselling Information
Advice Patients the Following1
For Ivabradine
Fetal Toxicity
Advise pregnant women of the potential risks to a fetus. Advise females of reproductive potential to use effective contraception and to notify their healthcare provider with a known or suspected pregnancy.
Low Heart Rate
Advise patients to report significant decreases in heart rate or symptoms such as dizziness, fatigue, or hypotension.
Atrial Fibrillation
Advise patients to report symptoms of atrial fibrillation, such as heart palpitations or racing, chest pressure, or worsened shortness of breath.
Phosphenes
Advise patients about the possible occurrence of luminous phenomena (phosphenes). Advise patients to use caution if they are driving or using machines in situations where sudden changes in light intensity may occur, especially when driving at night. Advise patients that phosphenes may subside spontaneously during continued treatment with ivabradine.
Drug Interactions
Advise patients to avoid ingestion of grapefruit juice and St. John's wort.
Intake with Food
Advise patients to take the tablet twice daily with food.
For Carvedilol
- Patients should take carvedilol with food.
- Patients should not interrupt or discontinue using carvedilol without a physician's advice.
- Patients with heart failure should consult their physician if they experience signs or symptoms of worsening heart failure such as weight gain or increasing shortness of breath
- Patients may experience a drop in blood pressure when standing, resulting in dizziness and, rarely, fainting. Patients should sit or lie down when these symptoms of lowered blood pressure occur.
- If experiencing dizziness or fatigue, patients should avoid driving or hazardous tasks.
- Patients should consult a physician if they experience dizziness or faintness, in case the dosage should be adjusted.
- Diabetic patients should report any changes in blood sugar levels to their physician.
- Contact lens wearers may experience decreased lacrimation.
Part IV: Summary
Carvedilol/ivabradine have a proven chemical-pharmaceutical quality and are considered an approvable fixed-dose combination. Both carvedilol and ivabradine are well known, established substances, which are used as a combination in clinical practice. The clinical data on concomitant use are considered sufficient to support the fixed-dose combination in patients with CHF who are on a stable fixed dose regimen with both monocomponents.
- Combining ivabradine to carvedilol in patients with stable IHF or patients with systolic HF in sinus rhythm with heart rate >70 bpm is associated with better heart rate reduction, improvement of quality of life, and reduced rehospitalisation.
- The early coadministration of ivabradine/β-blockers during a decompensation episode of HFrEF is feasible and safe. At 4 months ivabradine/β-blockers is associated with a significant improvement of functional and biochemical parameters which can be related to the prognosis, such as left ventricular ejection fraction, BNP levels, and severity of symptoms of HF.
- The addition of ivabradine to carvedilol in patients with CHF showed shorter β-blocker uptitration period, higher final β-blockers dose, greater heart rate reduction, and better exercise capacity.
Part V: References
- As per the CARLOC-I PI.
- COREG® (carvedilol) tablets. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2005/020297s013lbl.pdf Accessed on: 19th August 2021.
- COREG® (carvedilol) tablets. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/209964lbl.pdf Accessed on: 19th August 2021.
- Singh S and Preuss CV. Carvedilol. [Updated 2021 Feb 17]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK534868/
- Ivabradine. Available at: https://pubchem.ncbi.nlm.nih.gov/compound/Ivabradine#section=Biological-Half-Life Accessed on: 19th August 2021.
- Public Assessment Report Scientific discussion. Prescoriel. Available at: https://mri.cts-mrp.eu/Human/Downloads/NL_H_3548_005_PAR.pdf Accessed on: 19th August 2021.
- Al Saadi T; Sallam M, Al Hashmi K, et al. Effect of carvedilol versus carvedilol/ivabradine combination on heart rate, quality of life, morbidity and mortality in patients with stable ischemic heart failure.
- Bocchi EA, Böhm M, Borer JS, et al. Effect of Combining Ivabradine and β-Blockers: Focus on the Use of Carvedilol in the SHIFT Population. Cardiology. 2015;131:218–224.
- Hidalgo FJ, Anguita M, Castillo JC, et al. Effect of early treatment with ivabradine combined with beta blockers versus beta blockers alone in patients hospitalised with heart failure and reduced left ventricular ejection fraction (ETHIC-AHF): A randomised study. Int J Cardiology. 2016.
- Bagriy AE, Shchukina EV, Malovichko SI, et al. Addition of ivabradine to carvedilol reduces duration of carvedilol uptitration and improves exercise capacity in patients with chronic heart failure. J Am Coll Cardiol. 2013;61(10_Supplement)_E700.

.svg?iar=0&updated=20230109065058&hash=B8F025B8AA9A24E727DBB30EAED272C8)